Kyselina ferulová: Porovnání verzí
nový článek podle enwiki |
pokračování |
||
| Řádek 27: | Řádek 27: | ||
| P-věty = {{P|261}} {{P|264}} {{P|271}} {{P|280}} {{P|302+352}} {{P|304+340}} {{P|305+351+338}} {{P|312}} {{P|321}} {{P|332+313}} {{P|337+313}} {{P|362}} {{P|403+233}} {{P|405}} {{P|501}}<ref name=PubChem /> |
| P-věty = {{P|261}} {{P|264}} {{P|271}} {{P|280}} {{P|302+352}} {{P|304+340}} {{P|305+351+338}} {{P|312}} {{P|321}} {{P|332+313}} {{P|337+313}} {{P|362}} {{P|403+233}} {{P|405}} {{P|501}}<ref name=PubChem /> |
||
}} |
}} |
||
''Kyselina ferulová''' je [[organická sloučenina]] patřící mezi [[hydroxyskořicové kyseliny]]. Jedná se o fenolickou sloučeninu často přítomnou ve [[buněčná stěna|stěnách]] rostlinných buněk, kde bývá navázána na vedlejší řetězce molekul jako jsou [[arabinoxylan]]y. Tato kyselina je složkou ligninu a slouží jako prekurzor při výrobě dalších [[aromaticita|aromatických]] sloučenin. Název je odvozen od jejího výskytu v rostlinách rodu [[ločidlo]] (''[[Ferula]]''). |
|||
== Výskyt == |
|||
Kyselina ferulová je stavebním prvkem [[lignocelulóza|lignocelulóz]], jako jsou [[pektin]]y<ref>{{Citace periodika | autor1 = Luc Saulnier | autor2 = Jean-François Thibault | titul = Ferulic acid and diferulic acids as components of sugar-beet pectins and maize bran heteroxylans | periodikum = Journal of the Science of Food and Agriculture | datum vydání = 1999-03-01 | strany = 396–402 | doi = 10.1002/(SICI)1097-0010(19990301)79:3<396::AID-JSFA262>3.0.CO;2-B}}</ref> a [[lignin]]y. |
|||
=== V potravinách === |
|||
Kyselina ferulová se nachází v řadě druhů zeleniny, v poměrně vysokých koncentracích je přítomna v [[popcorn]]u a [[bambusové výhonky|bambusových výhoncích]].<ref>{{Citace periodika | autor1 = Zhaohui Zhao | autor2 = Mohammed H. Moghadasian | titul = Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review | periodikum = Food Chemistry | rok vydání = 2008 | strany = 691–702 | doi = 10.1016/j.foodchem.2008.02.039 | pmid = 26049981}}</ref><ref>{{Citace periodika | autor1 = Naresh Kumar | autor2 = Vikas Pruthi | titul = Potential applications of ferulic acid from natural sources | periodikum = Biotechnology Reports | rok vydání = 2014 | strany = 86–93 | doi = 10.1016/j.btre.2014.09.002 | pmid = 28626667}}</ref> Jde, společně s [[kyselina kávová|kyselinou kávovou]] a [[kyselina isoferulová|isoferulovou]], o hlavní metabolit [[kyselina chlorogenová|chlorogenových kyselin]] u lidí a absorbována je v [[tenké střevo|tenkém střevu]], zatímco ostatní metabolity kyseliny chlorogenové, jako jsou kyselina dihydroferulová a feruloylglycin, se absorbují působením [[střevní mikroflóra|střevní mikroflóry]] v [[tlusté střevo|tlustém střevu]].<ref>{{Citace monografie | autor1 = Debasis Bagchi | autor2 = Hiroyoshi Moriyama | autor3 = Anand Swaroop | titul = Green Coffee Bean Extract in Human Health | vydavatel = CRC Press | rok vydání = 2016 | strany = 92 | isbn = 9781315353982}}</ref> |
|||
<!-- In cereals, ferulic acid is localized in the [[bran]] – the hard outer layer of grain. In [[wheat]], phenolic compounds are mainly found in the form of insoluble bound ferulic acid and may be relevant to resistance to wheat fungal diseases.<ref>{{cite journal | doi = 10.1111/j.1365-2621.2005.01057.x | title = Effect of wheat variety, farming site, and bread-baking on total phenolics | date = 2006 | last1 = Gelinas | first1 = Pierre | last2 = McKinnon | first2 = Carole M. | journal = International Journal of Food Science and Technology | volume = 41 | issue = 3 | pages = 329–332}}</ref> The highest known concentration of ferulic acid [[glucoside]] has been found in [[flaxseed]] ({{val|4.1|0.2|u=g/kg}}).<ref>{{cite journal | doi = 10.1002/pca.973 | pmid = 17623361 | title = Microwave-assisted extraction of the main phenolic compounds in flaxseed | date = 2007 | last1 = Beejmohun | first1 = Vickram | last2 = Fliniaux | first2 = Ophélie | journal = Phytochemical Analysis | volume = 18 | issue = 4 | pages = 275–285}}</ref> It is also found in [[barley]] grain.<ref>{{cite journal|title=Phenolic Compounds of Barley Grain and Their Implication in Food Product Discoloration|first1=Zory|last1=Quinde-Axtell|first2=Byung-Kee|last2=Baik|journal=J. Agric. Food Chem.|date=2006|volume=54|issue=26|pages=9978–84|doi=10.1021/jf060974w|pmid=17177530}}</ref> |
|||
[[Asterid]] [[eudicot]] plants can also produce ferulic acid. The tea brewed from the leaves of [[yacón]] (''Smallanthus sonchifolius''), a plant traditionally grown in the northern and central [[Andes]], contains quantities of ferulic acid. In [[legume]]s, the white bean variety [[navy bean]] is the richest source of ferulic acid among the common bean (''[[Phaseolus vulgaris]]'') varieties.<ref>{{cite journal | last1 = Luthria | first1 = Devanand L. | last2 = Pastor-Corrales | first2 = Marcial A. | date = 2006 | title = Phenolic acids content of fifteen dry edible bean (''Phaseolus vulgaris'' L.) varieties | journal = Journal of Food Composition and Analysis | volume = 19 | issue = 2–3| pages = 205–211 | doi=10.1016/j.jfca.2005.09.003}}</ref> It is also found in [[horse gram]]s (''Macrotyloma uniflorum'').{{Citation needed|date=December 2019|reason=removed citation to predatory publisher content}} |
|||
Although there are many sources of ferulic acid in nature, its [[bioavailability]] depends on the form in which it is present: free ferulic acid has limited solubility in water, and hence poor bioavailability. In wheat grain, ferulic acid is found bound to [[cell wall]] [[polysaccharide]]s, allowing it to be released and absorbed in the small intestine.<ref>{{cite journal | doi = 10.1016/j.jcs.2008.12.001 | title =Bioavailability of ferulic acid is determined by its bioaccessibility| date = 2009 | last1 = Anson | first1 = Nuria Mateo | last2 = van den Berg | first2 = Robin | last3 = Bast | first3 = Aalt | last4 = Haenen | first4 = Guido R. M. M. | journal = Journal of Cereal Science | volume = 49 | issue = 2 | pages = 296–300}}</ref> |
|||
===In herbal medicines=== |
|||
Ferulic acid has been identified in [[Chinese medicine]] herbs such as ''[[Angelica sinensis]]'' (female ginseng), ''[[Cimicifuga|Cimicifuga heracleifolia]]''<ref>{{cite journal|last1=Sakai|first1=S.|last2=Kawamata|first2=H.|last3=Kogure|first3=T.|last4=Mantani|first4=N.|last5=Terasawa|first5=K.|last6=Umatake|first6=M.|last7=Ochiai|first7=H.|title=Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264.7 cells|journal=Mediators of Inflammation|date=1999|volume=8|issue=3|pages=173–175|pmid=10704056|doi=10.1080/09629359990513|pmc=1781798}}</ref> and ''[[Ligusticum|Ligusticum chuangxiong]]''. It is also found in the tea brewed from the European centaury (''[[Centaurium erythraea]]''), a plant used as a medical herb in many parts of Europe.<ref>{{cite journal | doi = 10.1021/jf001145s | title = Antioxidant Activity of ''Centaurium erythraea'' Infusion Evidenced by Its Superoxide Radical Scavenging and Xanthine Oxidase Inhibitory Activity | date = 2001 | last1 = Valentão | first1 = P. | last2 = Fernandes | first2 = E. | last3 = Carvalho | first3 = F. | last4 = Andrade | first4 = P. B. | last5 = Seabra | first5 = R. M. | last6 = Bastos | first6 = M. L. | journal = Journal of Agricultural and Food Chemistry | volume = 49 | issue = 7 | pages = 3476–3479 | pmid = 11453794}}</ref> |
|||
=== In processed foods === |
|||
Cooked [[sweetcorn]] releases increased levels of ferulic acid.<ref>{{cite web| url = http://www.news.cornell.edu/releases/Aug02/CornLiu.bpf.html | title = Cooking sweet corn boosts its ability to fight cancer and heart disease by freeing healthful compounds, Cornell scientists find | publisher = Cornell News | access-date = 2009-09-07}}</ref> As [[plant sterol]] [[ester]]s, this compound is naturally found in [[rice bran oil]], a popular cooking oil in several Asian countries.<ref name="baileys">{{cite book |title=Bailey's Industrial Oil and Fat Products |last=Orthoefer |first=F. T. |edition=6th |volume=2 |editor1-first=F. |editor1-last=Shahidi |date=2005 |publisher=John Wiley & Sons, Inc. |isbn=978-0-471-38552-3 |page=465 |chapter=Chapter 10: Rice Bran Oil |chapter-url=https://books.google.com/books?id=wG-0QgAACAAJ |access-date=2012-03-01}}</ref> |
|||
Ferulic acid [[glycoside|glucoside]] can be found in commercial [[bread]]s containing [[flaxseed]].<ref>{{cite journal | doi = 10.1016/j.foodchem.2008.02.088 | pmid = 26047292 | title = Phenolic glucosides in bread containing flaxseed | date = 2008 | last1 = Strandås | first1 = C. | last2 = Kamal-Eldin | first2 = A. | last3 = Andersson | first3 = R. | last4 = Åman | first4 = P. | journal = Food Chemistry | volume = 110 | issue = 4 | pages = 997–999}}</ref> [[Rye bread]] contains [[ferulic acid dehydrodimer]]s.<ref>{{cite journal|last1=Boskov Hansen|first1=H.|last2=Andreasen|first2=M.|last3=Nielsen|first3=M.|last4=Larsen|first4=L.|last5=Knudsen|first5=Bach K.|last6=Meyer|first6=A.|last7=Christensen|first7=L.|last8=Hansen|first8=Å.|title=Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making|journal=European Food Research and Technology|volume=214|issue=1|date=2014|pages=33–42|issn=1438-2377|doi=10.1007/s00217-001-0417-6|s2cid=85239461}}</ref> |
|||
== Metabolism == |
|||
[[File:CaffeicAcIn.png|thumb|300px|left|In plants, ferulic acid (right) is derived from [[phenylalanine]], which is converted to [[4-hydroxycinnamic acid]] (left) and then [[caffeic acid]].]] |
|||
=== Biosynthesis === |
|||
Ferulic acid is biosynthesized in plants from [[caffeic acid]] by the action of the enzyme [[caffeate O-methyltransferase|caffeate ''O''-methyltransferase]].<ref>{{cite book |last1= Shahadi |first1=Fereidoon|last2=Naczk |first2=Marian|title= Phenolics in Food and Nutraceuticals|url= https://archive.org/details/phenolicsfoodnut00shah_938 |url-access= limited |publisher= CRC Press|location= Florida|isbn= 978-1-58716-138-4|page= [https://archive.org/details/phenolicsfoodnut00shah_938/page/n12 4] |date= 2004}}</ref> |
|||
Ferulic acid, together with [[dihydroferulic acid]], is a component of [[lignocellulose]], serving to crosslink the lignin and polysaccharides, thereby conferring rigidity to the cell walls.<ref>{{cite journal|last1=Iiyama|first1=K.|last2=Lam|first2=T. B.-T.|last3=Stone|first3=B. A.|title=Covalent Cross-Links in the Cell Wall|journal=Plant Physiology|volume=104|issue=2|date=1994|pages=315–320|issn=0032-0889|doi=10.1104/pp.104.2.315|pmid=12232082|pmc=159201}}</ref> |
|||
It is an intermediate in the synthesis of [[monolignol]]s, the monomers of [[lignin]], and is also used for the synthesis of [[lignan]]s. |
|||
=== Biodegradation === |
|||
Ferulic acid is converted by certain strains of yeast, notably strains used in brewing of [[wheat beer]]s, such as [[Torulaspora delbrueckii|''Saccharomyces delbrueckii'']] (''Torulaspora delbrueckii''), to [[4-vinyl guaiacol]] (2-methoxy-4-vinylphenol) which gives beers such as [[Weissbier]] and Wit their distinctive clove-like flavour. ''[[Saccharomyces cerevisiae]]'' (dry baker's yeast) and ''[[Pseudomonas fluorescens]]'' are also able to convert ''trans''-ferulic acid into 2-methoxy-4-vinylphenol.<ref>{{Cite journal |
|||
| last1 = Huang | first1 = Z. |
|||
| last2 = Dostal | first2 = L. |
|||
| last3 = Rosazza | first3 = J. P. |
|||
| title = Microbial transformations of ferulic acid by ''Saccharomyces cerevisiae'' and ''Pseudomonas fluorescens '' |
|||
| journal = Applied and Environmental Microbiology |
|||
| volume = 59 |
|||
| issue = 7 |
|||
| pages = 2244–2250 |
|||
| date = 1993 |
|||
| doi = 10.1128/AEM.59.7.2244-2250.1993 |
|||
| pmid = 8395165 |
|||
| pmc = 182264 |
|||
}}</ref> In ''P. fluorescens'', a [[ferulic acid decarboxylase]] has been isolated.<ref>{{Cite journal |
|||
| last1 = Huang | first1 = Z. |
|||
| last2 = Dostal | first2 = L. |
|||
| last3 = Rosazza | first3 = J. P. |
|||
| title = Purification and characterization of a ferulic acid decarboxylase from ''Pseudomonas fluorescens'' |
|||
| journal = Journal of Bacteriology |
|||
| volume = 176 |
|||
| issue = 19 |
|||
| pages = 5912–5918 |
|||
| date = 1994 |
|||
| pmid = 7928951 |
|||
| pmc = 196807 |
|||
| doi=10.1128/jb.176.19.5912-5918.1994 |
|||
}}</ref> |
|||
== Ecology == |
|||
Ferulic acid is one of the compounds that initiate the ''vir'' (virulence) region of ''[[Agrobacterium tumefaciens]]'', inducing it to infect plant cells.<ref>{{cite journal|last1=Kalogeraki|first1=Virginia S.|last2=Zhu|first2=Jun|last3=Eberhard|first3=Anatol|last4=Madsen|first4=Eugene L.|last5=Winans|first5=Stephen C.|title=The phenolic ''vir'' gene inducer ferulic acid is ''O''-demethylated by the VirH2 protein of an ''Agrobacterium tumefaciens'' Ti plasmid|journal=Molecular Microbiology|date=November 1999|volume=34|issue=3|pages=512–522|doi=10.1046/j.1365-2958.1999.01617.x|pmid=10564493|s2cid=28658847|doi-access=free}}</ref> |
|||
== Extraction == |
|||
It can be extracted from wheat bran and maize bran using concentrated alkali.<ref>{{cite journal|last1=Buranov|first1=Anvar U.|first2=G.|last2=Mazza|title=Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents|journal=Food Chemistry|date=2009|volume=115|issue=4|pages=1542–1548|doi=10.1016/j.foodchem.2009.01.059}}</ref> |
|||
[[File:Ferulicacidspectrum.PNG|thumb|right|[[Ultraviolet–visible spectroscopy|UV–visible spectrum]] of ferulic acid, with ''λ''<sub>max</sub> at 321 nm and a shoulder at 278 nm]] |
|||
== Other applications == |
|||
=== Mass spectrometry === |
|||
It is used as a matrix for [[protein]]s in [[Matrix-assisted laser desorption/ionization|MALDI]] [[mass spectrometry]] analyses.<ref>{{Cite journal |
|||
| last1 = Beavis | first1 = R. C. |
|||
| last2 = Chait | first2 = B. T. |
|||
| last3 = Fales | first3 = H. M. |
|||
| doi = 10.1002/rcm.1290031207 |
|||
| title = Cinnamic acid derivatives as matrices for ultraviolet laser desorption mass spectrometry of proteins |
|||
| journal = Rapid Communications in Mass Spectrometry |
|||
| volume = 3 |
|||
| issue = 12 |
|||
| pages = 432–435 |
|||
| date = 1989 |
|||
| pmid = 2520223 |
|||
| bibcode = 1989RCMS....3..432B |
|||
}}</ref> |
|||
== See also == |
|||
* [[Caffeic acid]] |
|||
* [[Coumaric acid]] |
|||
* [[Diferulic acids]] |
|||
* [[Eugenol]] |
|||
* [[Isoferulic acid]], an isomer of ferulic acid |
|||
== References == |
|||
<ref>{{cite journal |first1=Catherine |last1=Tomaro-Duchesneau |first2=Shyamali |last2=Saha |first3=Meenakshi |last3=Malhotra |first4=Michael |last4=Coussa-Charley |first5=Imen |last5=Kahouli |first6=Mitchell L. |last6=Jones |first7=Alain |last7=Labbe |first8=Satya |last8=Prakash | date = 2012 | title = Probiotic Ferulic Acid Esterase Active ''Lactobacillus fermentum'' NCIMB 5221 APA Microcapsules for Oral Delivery: Preparation and In Vitro Characterization | journal = Pharmaceuticals | volume = 5 | pages = 236–248 | issue = 2 | doi = 10.3390/ph5020236 | pmid=24288090 | pmc=3763630}}</ref> --> |
|||
[[Kategorie:Aromatické hydroxykyseliny|ferulová]] |
[[Kategorie:Aromatické hydroxykyseliny|ferulová]] |
||
[[Kategorie:Nenasycené karboxylové kyseliny|ferulová]] |
[[Kategorie:Nenasycené karboxylové kyseliny|ferulová]] |
||
[[Kategorie:Fenylpropanoidy]] |
[[Kategorie:Fenylpropanoidy]] |
||
| ⚫ | |||
[[Kategorie:Fenolové antioxidanty]] |
[[Kategorie:Fenolové antioxidanty]] |
||
| ⚫ | |||
Verze z 31. 3. 2021, 12:11
| Kyselina ferulová | |
|---|---|
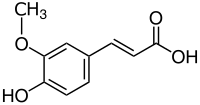 Strukturní vzorec | |
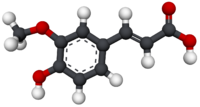 Model molekuly | |
| Obecné | |
| Systematický název | kyselina (2E)-3-(4-hydroxy-3-methoxyfenyl)prop-2-enová |
| Sumární vzorec | C10H10O4 |
| Vzhled | krystalický prášek |
| Identifikace | |
| Registrační číslo CAS | 1135-24-6 |
| EC-no (EINECS/ELINCS/NLP) | 214-490-0 |
| PubChem | 445858 |
| SMILES | COC1=C(C=CC(=C1)C=CC(=O)O)O |
| InChI | 1S/C10H10O4/c1-14-9-6-7(2-4-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/b5-3+ |
| Vlastnosti | |
| Molární hmotnost | 194,18 g/mol |
| Teplota tání | 174 °C (447 K)[1] |
| Disociační konstanta pKa | 4,42[1] |
| Rozpustnost ve vodě | 0,597 g/100 ml[1] |
| Rozpustnost v polárních rozpouštědlech | rozpustná v ethanolu a ethylacetátu[1] |
| Rozpustnost v nepolárních rozpouštědlech | rozpustná v benzenu a diethyletheru[1] |
| Bezpečnost | |
| [1] | |
| H-věty | H315 H319 H335[1] |
| P-věty | P261 P264 P271 P280 P302+352 P304+340 P305+351+338 P312 P321 P332+313 P337+313 P362 P403+233 P405 P501[1] |
Některá data mohou pocházet z datové položky. | |
Kyselina ferulová' je organická sloučenina patřící mezi hydroxyskořicové kyseliny. Jedná se o fenolickou sloučeninu často přítomnou ve stěnách rostlinných buněk, kde bývá navázána na vedlejší řetězce molekul jako jsou arabinoxylany. Tato kyselina je složkou ligninu a slouží jako prekurzor při výrobě dalších aromatických sloučenin. Název je odvozen od jejího výskytu v rostlinách rodu ločidlo (Ferula).
Výskyt
Kyselina ferulová je stavebním prvkem lignocelulóz, jako jsou pektiny[2] a ligniny.
V potravinách
Kyselina ferulová se nachází v řadě druhů zeleniny, v poměrně vysokých koncentracích je přítomna v popcornu a bambusových výhoncích.[3][4] Jde, společně s kyselinou kávovou a isoferulovou, o hlavní metabolit chlorogenových kyselin u lidí a absorbována je v tenkém střevu, zatímco ostatní metabolity kyseliny chlorogenové, jako jsou kyselina dihydroferulová a feruloylglycin, se absorbují působením střevní mikroflóry v tlustém střevu.[5]
- ↑ a b c d e f g h https://pubchem.ncbi.nlm.nih.gov/compound/445858
- ↑ Luc Saulnier; Jean-François Thibault. Ferulic acid and diferulic acids as components of sugar-beet pectins and maize bran heteroxylans. Journal of the Science of Food and Agriculture. 1999-03-01, s. 396–402. DOI 10.1002/(SICI)1097-0010(19990301)79:3<396::AID-JSFA262>3.0.CO;2-B.
- ↑ Zhaohui Zhao; Mohammed H. Moghadasian. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chemistry. 2008, s. 691–702. DOI 10.1016/j.foodchem.2008.02.039. PMID 26049981.
- ↑ Naresh Kumar; Vikas Pruthi. Potential applications of ferulic acid from natural sources. Biotechnology Reports. 2014, s. 86–93. DOI 10.1016/j.btre.2014.09.002. PMID 28626667.
- ↑ Debasis Bagchi; Hiroyoshi Moriyama; Anand Swaroop. Green Coffee Bean Extract in Human Health. [s.l.]: CRC Press, 2016. ISBN 9781315353982. S. 92.

