2-pyridon: Porovnání verzí
m odebrána Kategorie:2-pyridony; přidána Kategorie:2-Pyridony za použití HotCat |
pokračování |
||
| Řádek 31: | Řádek 31: | ||
| P-věty = {{P|261}} {{P|264}} {{P|270}} {{P|271}} {{P|280}} {{P|301+310}} {{P|302+352}} {{P|304+340}} {{P|305+351+338}} {{P|312}} {{P|321}} {{P|330}} {{P|332+313}} {{P|337+313}} {{P|362}} {{P|403+233}} {{P|405}} {{P|501}}<ref name=pubchem /> |
| P-věty = {{P|261}} {{P|264}} {{P|270}} {{P|271}} {{P|280}} {{P|301+310}} {{P|302+352}} {{P|304+340}} {{P|305+351+338}} {{P|312}} {{P|321}} {{P|330}} {{P|332+313}} {{P|337+313}} {{P|362}} {{P|403+233}} {{P|405}} {{P|501}}<ref name=pubchem /> |
||
}} |
}} |
||
'''2-pyridon''' je [[organická sloučenina]] se vzorcem C<sub>5</sub>H<sub>4</sub>NH(O), za standardních podmínek bezbarvá pevná látka. Vytváří [[dimer]]y spojené [[vodíková vazba|vodíkovými vazbami]] a existuje ve dvojici tautomerů. |
|||
== Tautomerie == |
|||
[[Soubor:2-pyridone-chemical-tautomer.svg|200px|left|Tautomerie u 2-pyridonu]] |
|||
Druhou [[tautomerie|tautomerní]] formou 2-pyridonu je 2-hydroxypyridin. Obdobná [[laktam]]-[[laktim]]ová tautomerie se vyskytuje i u mnoha odvozených sloučenin.<ref name=forlani/> |
|||
=== Tautomerie v pevném skupenství === |
|||
[[Amidy|Amidová]] skupina [vytváří vodíkové vazby s ostatními sloučeninami obsahujícími [[dusík]] a [[kyslík]]. |
|||
V pevném skupenství převažuje 2-pyridon, což bylo potvrzeno [[rentgenová krystalografie|rentgenovou krystalografií]], kde se ukázalo, že vodík se v pevném skupenství nachází blíže k dusíku než ke kyslíku (v důsledku nízké elektronové hustoty na vodíku je přesné určení obtížné), a [[infračervená spektroskopie|infračervenou spektroskopií]], kde byly patrné krekvence odppovídající vazbám C=O, zatímco frekvence příslušící vazbám O-H nebyly zaznamenány.<ref name=Yang>{{Citace periodika | autor1 = H. W. Yang | autor2 = B. M. Craven | titul = Charge Density of 2-Pyridone | periodikum = [[Acta Crystallographica|Acta Crystallographica B]] | rok vydání = 1998 | strany = 912–920 | doi = 10.1107/S0108768198006545 | pmid= 9880899}}</ref><ref name=Penfold>{{Citace periodika | autor = B. R. Penfold | titul = The Electron Distribution in Crystalline Alpha Pyridone | periodikum = Acta Crystallographica | rok vydání = 1953 | strany = 591–600 | doi = 10.1107/S0365110X5300168X}}</ref><ref name=Ohms>{{Citace periodika | autor1 = U. Ohms | autor2 = H. Guth | autor3 = E. Heller | autor4 = H. Dannöhl | autor5 = A. Schweig | titul = Comparison of Observed and Calculated Electron-Density 2-Pyridone, C<sub>5</sub>H<sub>5</sub>NO, Crystal-Structure Refinements at 295K and 120K, Experimental and Theoretical Deformation Density Studies | periodikum = [[Zeitschrift für Kristallographie – Crystalline Materials|Zeitschrift für Kristallographie]] | rok vydání = 1984 | strany = 185–200 | doi = 10.1524/zkri.1984.169.14.185}}</ref><ref name=Almlof>{{Citace periodika | autor1 = J. Almlöf | autor2 = A. Kvick | autor3 = I. Olovsson | titul = Hydrogen Bond Studies Crystal Structure of Intermolecular Complex 2-Pyridone-6-Chloro-2-Hdroxypyridine | periodikum = Acta Crystallographica B | rok vydání = 1971 | strany = 1201–1208 | doi = 10.1107/S0567740871003753}}</ref> |
|||
=== Tautomerie v roztoku === |
|||
<!-- The determination of which of the two tautomeric forms is present in [[Solution (chemistry)|solution]] has been the subject of many publications. The energy difference appears to be very small and is dependent on the [[Chemical polarity|polarity]] of the [[solvent]]. [[Chemical polarity|Non-polar solvents]] favour the formation of 2-hydroxypyridine whereas [[Chemical polarity|polar solvents]] such as [[alcohols]] and [[water]] favour the formation of 2-pyridone.<ref name ="forlani"> |
|||
{{cite journal |author1=Forlani L. |author2=Cristoni G. |author3=Boga C. |author4=Todesco P. E. |author5=Del Vecchio E. |author6=Selva S. |author7=Monari M. |title=Reinvestigation of tautomerism of some substituted 2-hydroxypyridines |journal=[[Arkivoc]] |year=2002 |pages=198–215 |volume=XI |issue=11 |doi=10.3998/ark.5550190.0003.b18 |url=http://arkat-usa.org/home.aspx?VIEW=MANUSCRIPT&MSID=590&SEARCH=2-hydroxypyridines |doi-access=free }}{{Dead link|date=March 2019 |bot=InternetArchiveBot |fix-attempted=yes }} |
|||
</ref><ref>{{cite journal |
|||
|author1=Vögeli U. |author2=von Philipsborn W. | title=C-13 and H-1 NMR Spectroscopie Studies on Structure of N-Methyle-3-Pyridone and 3-Hydroypyridine | journal= Org Magn Reson| year=1973| pages= 551–559| doi=10.1002/mrc.1270051202| volume= 5 |
|||
| issue= 12}}</ref><ref>{{cite journal|author1=Specker H. |author2=Gawrosch H. | title=Ultraviolet absorption of benztriaxole, pryridone and its salts | journal= [[Chem. Ber.]]| year=1942| pages= 1338–1348| issue=75|doi=10.1002/cber.19420751115 }}</ref><ref>{{cite journal|author1=Leis D. G. |author2=Curran B. C. | title = Electric Moments of Some Gamma-Substituted Pyridines | journal=[[Journal of the American Chemical Society]] | year=1945 | pages=79–81 | issue=1 |doi = 10.1021/ja01217a028| volume= 67}}</ref><ref>{{cite journal|author1=Albert A. |author2=Phillips J. N. | title=Ionisation Constants of Heterocyclic Substances Hydroxy-Derivates of Nitrogenous Six-Membered Ring-Compounds| journal=[[J. Chem. Soc.]] | year=1956| pages= 1294–1304| doi=10.1039/jr9560001294}}</ref><ref>{{cite journal|author1=Cox R. H. |author2=Bothner-By A. A | title=Proton Magnetic Resonance Spectra of Tautomeric Substituted Pyridines and Their Conjugated Acides | journal=J. Phys. Chem. | year=1969 | pages=2465–2468 | issue=8|doi=10.1021/j100842a001|volume= 73 }}</ref><ref>{{cite journal|author=Aksnes DW, Kryvi | title=Substituent and Solvent Effects in Proton Magnetic -Resonance (PMR) Spectra of 6 2-Substituted Pyridines| journal=Acta Chem. Scand. | year=1972| pages=2255–2266 | issue=26 |doi=10.3891/acta.chem.scand.26-2255|volume=26|last2=Kryvi|first2=Håkon|last3=Samuelson|first3=Olof|last4=Sjöstrand|first4=Elisabeth|last5=Svensson|first5=Sigfrid|doi-access=free}}</ref><ref>{{cite journal|vauthors=Aue DH, Betowski LD, Davidson WR, Bower MT, Beak P | title=Gas-Phase Basicities of Amides and Imidates - Estimation of Protomeric Equilibrium-Constantes by the Basicity methode in the Gas-Phase | journal=[[Journal of the American Chemical Society]] | year=1979 | pages=1361–1368 | issue=6|doi=10.1021/ja00500a001| volume= 101}}</ref><ref>{{cite journal| author=Frank J., [[Alan R. Katritzky]] | title= Tautomeric pyridines. XV. Pyridone-hydroxypyridine equilibria in solvents of different polarity| journal= J Chem Soc Perkin Trans 2 | year=1976 | pages=1428–1431 | doi= 10.1039/p29760001428| issue=12}}</ref> |
|||
The energy difference for the two tautomers in the gas phase was measured by [[Infrared spectroscopy|IR-spectroscopy]] to be 2.43 to 3.3 [[Joule|kJ]]/[[mole (unit)|mol]] for the solid state and 8.95 kJ/mol and 8.83 kJ/mol for the liquid state.<ref>{{cite journal |
|||
|author1=Brown R. S. |author2=Tse A. |author3=Vederas J. C. | title=Photoelectro-Determined Core Binding Energies and Predicted Gas-Phase Basicities for the 2-Hydroxypyridine 2-Pyridone System | journal=[[Journal of the American Chemical Society]] | year= 1980| pages= 1174–1176| issue=3|doi=10.1021/ja00523a050 |
|||
| volume=102}} |
|||
</ref><ref>{{cite journal |
|||
| author= Beak P.| title=Energies and Alkylation of Tautomeric Heterocyclic-Compounds - Old Problems New Answers | journal=[[Acc. Chem. Res.]] | year=1977 | pages=186–192 | issue=5|doi=10.1021/ar50113a006 |
|||
| volume= 10}} |
|||
</ref><ref name="Abdulla">{{cite journal|author1=Abdulla H. I. |author2=El-Bermani M. F. | title=Infrared studies of tautomerism in 2-hydroxypyridine 2-thiopyridine and 2-aminopyridine | journal=Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy| year=2001 | pages=2659–2671 | issue=13|doi=10.1016/S1386-1425(01)00455-3|pmid=11765793 | volume= 57|bibcode=2001AcSpA..57.2659A}} |
|||
</ref> |
|||
===Tautomerisation mechanism A=== |
|||
The single molecular tautomerisation has a forbidden [[rearrangement reaction|1-3 suprafacial]] [[transition state]] and therefore has a high [[energy barrier]] for this [[tautomer]]isation, which was calculated with [[theoretical chemistry|theoretical methods]] to be 125 or 210 kJ/mol. The direct tautomerisation is energetically not favoured. There are other possible mechanisms for this tautomerisation.<ref name="Abdulla" /> |
|||
==Dimerisation== |
|||
[[Image:2-pyridone dimer.svg|200px|[[Dimer (chemistry)|dimer]]]] |
|||
2-Pyridone and 2-hydroxypyridine can form dimers with two hydrogen bonds.<ref name="Hammes">{{cite journal|vauthors=Hammes GG, Lillford PJ | title=A Kinetic and Equilibrium Study of Hydrogen Bond Dimerization of 2-Pyridone in Hydrogen Bonding Solvent | journal=[[J. Am. Chem. Soc.]] | year=1970 | pages=7578–7585 | issue=26| doi=10.1021/ja00729a012| volume= 92}}</ref> |
|||
===Aggregation in the solid state=== |
|||
In the solid state the dimeric form is not present; the 2-pyridones form a helical structure over hydrogen bonds. Some substituted 2-pyridones form the dimer in solid state, for example the 5-methyl-3-carbonitrile-2-pyridone. The determination of all these structures was done by [[X-ray crystallography]]. |
|||
In the solid state the hydrogen is located closer to the oxygen so it could be considered to be right to call the colourless crystals in the flask 2-pyridone.<ref name ="forlani" /><ref name="Yang" /><ref name="Penfold" /><ref name="Ohms" /><ref name="Almlof" /> |
|||
===Aggregation in solution=== |
|||
In solution the dimeric form is present; the ratio of dimerisation is strongly dependent on the polarity of the solvent. Polar and protic solvents interact with the [[hydrogen bonds]] and more [[monomer]] is formed. [[Hydrophobic]] effects in [[Chemical polarity|non-polar solvents]] lead to a predominance of the dimer. The ratio of the tautomeric forms is also dependent on the solvent. All possible tautomers and dimers can be present and form an equilibrium, and the exact measurement of all the [[equilibrium constants]] in the system is extremely difficult.<ref name="Hammes" /><ref name="Gilchrist" /><ref name="Rybakov" /><ref name="Guareschi" /><ref name="Baron" /><ref name="Fischer" /><ref name="Zipse" /><ref name="Rawson" /><ref name="Shima" /><ref name="Gerald" /> |
|||
([[NMR spectroscopy|NMR-spectroscopy]] is a slow method, high resolution [[Infrared spectroscopy|IR-spectroscopy]] in solvent is difficult, the broad absorption in [[UV/VIS spectroscopy|UV-spectroscopy]] makes it hard to discriminate 3 and more very similar [[molecules]]). |
|||
Some publications only focus one of the two possible patterns, and neglect the influence of the other. For example, to calculation of the energy difference of the two tautomers in a non-polar solution will lead to a wrong result if a large quantity of the substance is on the side of the dimer in an equilibrium. |
|||
===Tautomerisation mechanism B=== |
|||
The direct tautomerisation is not energetically favoured, but a [[Dimer (chemistry)|dimerisation]] followed by a double proton transfer and [[Dissociation (chemistry)|dissociation]] of the dimer is a self catalytic path from one tautomer to the other. Protic solvents also mediate the proton transfer during the tautomerisation. |
|||
==Synthesis== |
|||
2-Pyrone can be obtained by a cyclisation reaction, and converted to 2-pyridone via an exchange reaction with [[ammonia]]: |
|||
:[[Image:2-pyridone-chemical-synthesis-1.png|350px|2-Pyridone synthesis from 2-pyran]] |
|||
Pyridine forms an [[Amine oxide|''N''-oxide]] with some oxidation agents such as [[hydrogen peroxide]]. This [[Amine oxide|pyridine-''N''-oxide]] undergoes a rearrangement reaction to 2-pyridone in [[acetic anhydride]]:<ref name="Katada">{{cite journal |title=Pyridin-N-oxydと酸無水物との反應|trans-title=Reaction between Pyridin-N-oxyd and acid anhydride | doi = 10.1248/yakushi1947.67.3-4_51|language= ja|year= 1947|journal= Yakugaku Zasshi|volume= 67|issue= 3–4|pages= 51–52|doi-access= free}}</ref><ref name="Ochiai">{{cite journal | doi =10.1021/jo01133a010| title =Recent Japanese Work on the Chemistry of Pyridine 1-Oxide and Related Compounds| journal =The Journal of Organic Chemistry| volume =18| issue =5| pages =534–551| year =1953| last1 =Ochiai| first1 =Eiji.}}</ref><ref name="BOEKELHEIDE">{{cite journal | doi =10.1021/jo01061a037| title =The Rearrangement of Substituted Pyridine N-Oxides with Acetic Anhydride1.2| journal =The Journal of Organic Chemistry| volume =26| issue =2| pages =428–430| year =1961| last1 =Boekelheide| first1 =V.| last2 =Lehn| first2 =W. L.}}</ref> |
|||
:[[Image:2-pyridone-chemical-synthesis.svg|250px|2-Pyridone synthesis from pyridine-N-oxide]] |
|||
In the '''Guareschi-Thorpe condensation''' [[cyanoacetamide]] reacts with a [[1,3-diketone]] to a [[2-pyridone]].<ref name="Gilchrist">Gilchrist, T.L. (1997). Heterocyclic Chemistry {{ISBN|0-470-20481-8}}</ref><ref name="Rybakov">{{cite journal |author1=Rybakov V. R. |author2=Bush A. A. |author3=Babaev E. B. |author4=Aslanov L. A. | title= 3-Cyano-4,6-dimethyl-2-pyridone (Guareschi Pyridone)| journal= Acta Crystallogr E | year=2004 | pages=o160–o161 | volume=6 |doi=10.1107/S1600536803029295 | issue= 2 }}</ref> The reaction is named after [[Icilio Guareschi]] and [[Jocelyn Field Thorpe]].<ref name="Guareschi">{{cite journal | author= I. Guareschi| title= Mem. Reale Accad. Sci. Torino II | volume= 46, 7, 11, 25 | year= 1896}}</ref><ref name="Baron">{{cite journal |author1=Baron, H. |author2=Remfry, F. G. P. |author3=Thorpe, J. F. | title=CLXXV.-The formation and reactions of imino-compounds. Part I. Condensation of ethyl cyanoacetate with its sodium derivative| journal = J. Chem. Soc., Trans. | volume= 85 | pages=1726–1761 | year= 1904 | doi=10.1039/ct9048501726|url=https://zenodo.org/record/1429705 }}</ref> |
|||
==Chemical properties== |
|||
===Catalytic activity=== |
|||
2-Pyridone catalyses a variety of proton-dependent reactions, for example the aminolysis of esters. In some cases, molten 2-pyridone is used as a solvent. The [[mutarotation]] of sugars and that 2-pyridone has a large effect on the reaction from activated esters with [[amine]]s in [[nonpolar]] [[solvent]], which is attributed to its tautomerisation and utility as a ditopic receptor. Current interest focuses on proton transfer from 2-pyridone and its tautomer, using [[isotope labeling]], [[chemical kinetics|kinetics]] and [[quantum chemical]] methods to determine the rate determining step in the reaction mechanism.<ref name="Fischer"> |
|||
{{cite journal |author1=Fischer C. B. |author2=Steininger H. |author3=Stephenson D. S. |author4=Zipse H. | title=Catalysis of Aminolysis of 4-Nitrophenyl Acetate by 2-Pyridone | journal= Journal for Physical Organic Chemistry | year= 2005 | pages= 901–907|volume =18|issue=9 |doi=10.1002/poc.914}}</ref><ref name="Zipse">{{cite journal|author1=L.-H. Wang|author2=H. Zipse|year=1996|title=Bifunctional Catalysis of Ester Aminolysis - A Computational and Experimental Study|journal=Liebigs Ann.|volume=1996|issue=10|pages=1501–1509|doi=10.1002/jlac.199619961003}}{{dead link|date=February 2019|bot=medic}}{{cbignore|bot=medic}}</ref><ref>{{cite journal|author1=Fischer C. B. |author2=Polborn K. |author3=Steininger H. |author4=Zipse H. | title= Synthesis and Solid-State Structures of Alkyl-Substituted 3-Cyano-2-pyridones| journal=Zeitschrift für Naturforschung |volume=59 | year=2004 | pages=1121–1131 |issue=59b|url = http://www.znaturforsch.com/sb/s59b1121.pdf | format= subscription required |doi=10.1515/znb-2004-1008 |s2cid=98273691 }} |
|||
</ref> |
|||
===Coordination chemistry=== |
|||
2-Pyridone and some [[Derivative (chemistry)|derivatives]] serve as [[ligands]] in coordination chemistry, usually as a 1,3-bridging ligand akin to [[Carboxylic acid|carboxylate]].<ref name="Rawson">{{cite journal |author1=Rawson J. M. |author2=Winpenny R. E. P. | title=The coordination chemistry of 2-pyridones and its derivatives| journal=Coordination Chemistry Reviews | year=1995 | pages=313–374| issue=139|doi=10.1016/0010-8545(94)01117-T | volume= 139}} |
|||
</ref> |
|||
===In nature=== |
|||
2-Pyridone is not naturally occurring, but a derivative has been isolated as a cofactor in certain [[hydrogenase]]s.<ref name="Shima">Shima, S.; Lyon, E. J.; Sordel-Klippert, M.; Kauss, M.; Kahnt, J.; Thauer, R. K.; Steinbach, K.; Xie, X.; Verdier, L. and Griesinger, C., "Structure elucidation: The cofactor of the iron-sulfur cluster free hydrogenase Hmd: structure of the light-inactivation product", Angew. Chem. Int. Ed., 2004, 43, 2547-2551.</ref> |
|||
==Environmental behavior== |
|||
2-Pyridone is rapidly degraded by microorganisms in the soil environment, with a half life less than one week.<ref name="Gerald">{{cite journal | doi = 10.2134/jeq1985.00472425001400040022x | url = http://jeq.scijournals.org/cgi/content/abstract/14/4/580 | pages = 580–584 | journal = Journal of Environmental Quality | title = Degradation of Pyridine Derivatives in Soil | volume = 14 | issue = 4 | author = Sims, Gerald K. | author2 = S | year = 1985 | url-status = dead | archive-url = https://archive.today/20080830100452/http://jeq.scijournals.org/cgi/content/abstract/14/4/580 | archive-date = 2008-08-30 }}</ref> Organisms capable of growth on 2-pyridone as a sole source of carbon, nitrogen, and energy have been isolated by a number of researchers. The most extensively studied 2-pyridone degrader is the gram positive bacterium ''[[Arthrobacter crystallopoietes]]'',<ref>{{cite journal | doi = 10.1007/BF00422519| pmid = 14106078 | pages = 137–153 | title = A crystalline pigment produced from 2-hydroxypyridine by arthrobacter crystallopoietes n.sp | year = 1963 | last1 = Ensign | first1 = Jerald C. | last2 = Rittenberg | first2 = Sydney C. | journal = Archiv für Mikrobiologie | volume = 47 | issue = 2| s2cid = 6389661 }}</ref> a member of the phylum [[Actinobacteria]] which includes numerous related organisms that have been shown to degrade pyridine or one or more alkyl-, carboxyl-, or hydroxyl-substituted pyridines. 2-Pyridone degradation is commonly initiated by mono-oxygenase attack, resulting in a diol, such as 2,5-dihydroxypyridine, which is metabolized via the maleamate pathway. Fission of the ring proceeds via action of 2,5-dihydroxypyridine monooxygenase, which is also involved in metabolism of nicotinic acid via the maleamate pathway. In the case of ''Arthrobacter crystallopoietes'', at least part of the degradative pathway is plasmid-borne.<ref name="LOU">{{cite journal | doi = 10.1080/10643388909388372 | url = http://www.mesg.anl.gov/Ed_web_files/oloughlinPDF_Files/CREC1989.pdf | last1 = Sims | first1 = G. K. | first2 = E.J. | last2 = O'Loughlin | last3 = Crawford | year = 1989 | first3 = Ronald | title = Degradation of pyridines in the environment | journal = CRC Critical Reviews in Environmental Control | volume = 19 | issue = 4 | pages = 309–340 | url-status = dead | archive-url = https://web.archive.org/web/20100527212347/http://www.mesg.anl.gov/Ed_web_files/oloughlinPDF_Files/CREC1989.pdf | archive-date = 2010-05-27 }}</ref> Pyridine diols undergo chemical transformation in solution to form intensely colored pigments. Similar pigments have been observed in [[quinoline]] degradation,<ref>{{cite journal | doi =10.1016/S0964-8305(96)00032-7| pages = 107–118 | title =Isolation, characterization, and substrate utilization of a quinoline-degrading bacterium | year =1996 | last1 =Oloughlin | first1 =E | last2 =Kehrmeyer | first2 =S | last3 =Sims | first3 =G | journal =International Biodeterioration & Biodegradation | volume =38 | issue =2}}</ref> also owing to transformation of metabolites, however the yellow pigments often reported in degradation of many pyridine solvents, such as unsubstituted [[pyridine]] or [[picoline]], generally result from overproduction of [[riboflavin]] in the presence of these solvents.<ref>{{cite journal | pages = 3423–3425 | pmid =16348793 | journal =Applied and Environmental Microbiology | title =Riboflavin Production during Growth of Micrococcus luteus on Pyridine | volume =58 | issue =10 | author =Sims, Gerald K. | author2 =O | year =1992 | pmc=183117| doi = 10.1128/AEM.58.10.3423-3425.1992 }}</ref> Generally speaking, degradation of pyridones, dihydroxypyridines, and pyridinecarboxylic acids is commonly mediated by oxygenases, whereas degradation of pyridine solvents often is not, and may in some cases involve an initial reductive step.<ref name="LOU"/> |
|||
==See also== |
|||
* [[2-Pyridone (data page)]] |
|||
* [[2-Pyrone]] |
|||
* [[4-Pyridone]] |
|||
*The 5-methyl-2-pyridone is used to make [[pirfenidone]]. |
|||
==References== |
|||
{{Reflist|2}} |
|||
# {{cite journal|author1=Engdahl K. |author2=Ahlberg P. | journal=Journal of Chemical Research | year=1977 | pages=340–341}} |
|||
# {{cite journal|vauthors=Bensaude O, Chevrier M, Dubois J | title=Lactim-Lactam Tautomeric Equilibrium of 2-Hydroxypyridines. 1.Cation Binding, Dimerization and Interconversion Mechanism in Aprotic Solvents. A Spectroscopic and Temperature-Jump Kinetic Study | journal=[[J. Am. Chem. Soc.]] | year=1978| pages=7055–7066 | doi=10.1021/ja00490a046| volume=100| issue=22}} |
|||
# {{cite journal|vauthors=Bensaude O, Dreyfus G, Dodin G, Dubois J | title=Intramolecular Nondissociative Proton Transfer in Aqueous Solutions of Tautomeric Heterocycles: a Temperature-Jump Kinetic Study | journal=[[J. Am. Chem. Soc.]] | year=1977| pages=4438–4446 | doi=10.1021/ja00455a037| volume=99| issue=13}} |
|||
# {{cite journal|vauthors=Bensaude O, Chevrier M, Dubois J | title=Influence of Hydration upon Tautomeric Equilibrium | journal=[[Tetrahedron Lett.]] | year=1978 | pages=2221–2224 | doi=10.1016/S0040-4039(01)86850-7| volume= 19| issue= 25}} |
|||
# {{cite journal|vauthors=Hammes GG, Park AC | title=Kinetic and Thermodynamic Studies of Hydrogen Bonding | journal=[[J. Am. Chem. Soc.]] | year=1969 | pages=956–961 | doi=10.1021/ja01032a028| volume=91| issue=4}} |
|||
# {{cite journal|vauthors=Hammes GG, Spivey HO | title=A Kinetic Study of the Hydrogen-Bond Dimerization of 2-Pyridone | journal=[[J. Am. Chem. Soc.]] | year=1966 | pages=1621–1625 | doi=10.1021/ja00960a006| issue= 8| volume= 88 | pmid=5942979}} |
|||
# {{cite journal|vauthors=Beak P, Covington JB, Smith SG | title=Structural Studies of Tautomeric Systems: the Importance of Association for 2-Hydroxypyridine-2-Pyridone and 2-Mercaptopyridine-2-Thiopyridone | journal=[[J. Am. Chem. Soc.]] | year=1976 | pages=8284–8286 | doi=10.1021/ja00441a079| volume=98| issue=25}} |
|||
# {{cite journal|vauthors=Beak P, Covington JB, White JM | title=Quantitave Model of Solvent Effects on Hydroxypyridine-Pyridone and Mercaptopyridine-Thiopyridone Equilibria: Correlation with Reaction-Field and Hydrogen-Bond Effects | journal=[[J. Org. Chem.]] | year=1980 | pages=1347–1353 | doi=10.1021/jo01296a001| volume= 45| issue= 8}} |
|||
# {{cite journal|vauthors=Beak P, Covington JB, Smith SG, White JM, Zeigler JM | title=Displacement of Protomeric Equilibria by Self-Association: Hydroxypyridine-Pyridone and Mercaptopyridine-Thiopyridone Isomer Pairs | journal=[[J. Org. Chem.]] | year=1980 | pages=1354–1362|doi= 10.1021/jo01296a002| volume= 45| issue= 8}} --> |
|||
[[Kategorie:2-Pyridony| ]] |
[[Kategorie:2-Pyridony| ]] |
||
Verze z 17. 7. 2021, 10:56
| 2-pyridon | |
|---|---|
 Strukturní vzorec laktimové formy | |
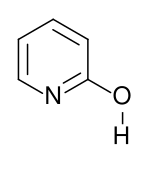 Strukturní vzorec laktamové formy | |
 Model molekuly laktamové formy | |
| Obecné | |
| Systematický název | pyridin-2(1H)-on |
| Sumární vzorec | C5H5NO |
| Vzhled | bezbarvá pevná látka |
| Identifikace | |
| Registrační číslo CAS | 142-08-5 |
| EC-no (EINECS/ELINCS/NLP) | 205-520-3 |
| PubChem | 8871 |
| ChEBI | 16540 |
| SMILES | Oc1ccccn1 (laktimová forma) C1=CC=CNC(=O)1 (laktamová forma) |
| InChI | 1S/C5H5NO/c7-5-3-1-2-4-6-5/h1-4H,(H,6,7) |
| Vlastnosti | |
| Molární hmotnost | 95,099 g/mol |
| Teplota tání | 107,8 °C (381,0 K)[1] |
| Teplota varu | 280 °C (553 K)[1] |
| Rozpustnost ve vodě | 100 g/100 ml (20 °C)[1] |
| Rozpustnost v polárních rozpouštědlech | rozpustný v methanolu a acetonu |
| Bezpečnost | |
| [1] | |
| H-věty | H301 H315 H319 H335[1] |
| P-věty | P261 P264 P270 P271 P280 P301+310 P302+352 P304+340 P305+351+338 P312 P321 P330 P332+313 P337+313 P362 P403+233 P405 P501[1] |
Některá data mohou pocházet z datové položky. | |
2-pyridon je organická sloučenina se vzorcem C5H4NH(O), za standardních podmínek bezbarvá pevná látka. Vytváří dimery spojené vodíkovými vazbami a existuje ve dvojici tautomerů.
Tautomerie

Druhou tautomerní formou 2-pyridonu je 2-hydroxypyridin. Obdobná laktam-laktimová tautomerie se vyskytuje i u mnoha odvozených sloučenin.[2]
Tautomerie v pevném skupenství
Amidová skupina [vytváří vodíkové vazby s ostatními sloučeninami obsahujícími dusík a kyslík.
V pevném skupenství převažuje 2-pyridon, což bylo potvrzeno rentgenovou krystalografií, kde se ukázalo, že vodík se v pevném skupenství nachází blíže k dusíku než ke kyslíku (v důsledku nízké elektronové hustoty na vodíku je přesné určení obtížné), a infračervenou spektroskopií, kde byly patrné krekvence odppovídající vazbám C=O, zatímco frekvence příslušící vazbám O-H nebyly zaznamenány.[3][4][5][6]
Tautomerie v roztoku
- ↑ a b c d e f https://pubchem.ncbi.nlm.nih.gov/compound/8871
- ↑ Chybná citace: Chyba v tagu
<ref>; citaci označenéforlaninení určen žádný text - ↑ H. W. Yang; B. M. Craven. Charge Density of 2-Pyridone. Acta Crystallographica B. 1998, s. 912–920. DOI 10.1107/S0108768198006545. PMID 9880899.
- ↑ B. R. Penfold. The Electron Distribution in Crystalline Alpha Pyridone. Acta Crystallographica. 1953, s. 591–600. DOI 10.1107/S0365110X5300168X.
- ↑ U. Ohms; H. Guth; E. Heller; H. Dannöhl; A. Schweig. Comparison of Observed and Calculated Electron-Density 2-Pyridone, C5H5NO, Crystal-Structure Refinements at 295K and 120K, Experimental and Theoretical Deformation Density Studies. Zeitschrift für Kristallographie. 1984, s. 185–200. DOI 10.1524/zkri.1984.169.14.185.
- ↑ J. Almlöf; A. Kvick; I. Olovsson. Hydrogen Bond Studies Crystal Structure of Intermolecular Complex 2-Pyridone-6-Chloro-2-Hdroxypyridine. Acta Crystallographica B. 1971, s. 1201–1208. DOI 10.1107/S0567740871003753.


