Dendrimer: Porovnání verzí
nový článek podle enwiki |
pokračování |
||
| Řádek 6: | Řádek 6: | ||
Molekuly dendrimerů jsou obvykle souměrné podle svých středů a často zaujímají kulovitý tvar. Většinou obsahují chemicky dostupnou funkční skupinu, nazývanou ohnisko nebo jádro. Rozdíly mezi dendrimery a dendrony jsou zobrazeny výše; tyto pojmy se však často zaměňují.<ref name=review>{{Citace periodika | autor1 = B. K. Nanjwade | autor2 = H. M. Bechra | autor3 = G. K. Derkar | autor4 = F. V. Manvi | autor5 = V. K. Nanjwade | titul = Dendrimers: emerging polymers for drug-delivery systems | periodikum = European Journal of Pharmaceutical Sciences | rok vydání = 2009 | strany = 185–196 | doi = 10.1016/j.ejps.2009.07.008 | pmid = 19646528}}</ref> |
Molekuly dendrimerů jsou obvykle souměrné podle svých středů a často zaujímají kulovitý tvar. Většinou obsahují chemicky dostupnou funkční skupinu, nazývanou ohnisko nebo jádro. Rozdíly mezi dendrimery a dendrony jsou zobrazeny výše; tyto pojmy se však často zaměňují.<ref name=review>{{Citace periodika | autor1 = B. K. Nanjwade | autor2 = H. M. Bechra | autor3 = G. K. Derkar | autor4 = F. V. Manvi | autor5 = V. K. Nanjwade | titul = Dendrimers: emerging polymers for drug-delivery systems | periodikum = European Journal of Pharmaceutical Sciences | rok vydání = 2009 | strany = 185–196 | doi = 10.1016/j.ejps.2009.07.008 | pmid = 19646528}}</ref> |
||
[[Soubor:Dendrimer ChemEurJ 2002 3858.jpg|thumb|Krystalová struktura polyfenylenového dendrimeru první generace<ref>{{Citace periodika | titul = Single-Crystal Structures of Polyphenylene Dendrimers | periodikum = Chemistry: A European Journal | rok vydání = 2002 | strany = 3858–3864 | doi = 10.1002/1521-3765(20020902)8:17<3858::AID-CHEM3858>3.0.CO;2-5}}</ref>]] |
|||
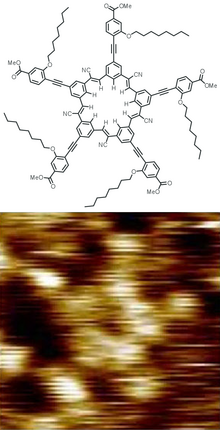
[[Soubor:Cyanostar STM.png|thumb|Struktura „cyanostarového“ dendrimeru a jeho obrázek ze [[skenovací tunelová mikroskopie|skenovacího tunelového mikroskopu]]<ref>{{Citace periodika | autor1 = B. E. Hirsch | autor2 = S. Lee | autor3 = B. Qiao | autor4 = C. H. Chen | autor5 = K. P. McDonald | autor6 = S. L. Tait | autor7 = A. H. Flood | titul = Anion-induced dimerization of 5-fold symmetric cyanostars in 3D crystalline solids and 2D self-assembled crystals | periodikum = [[ChemComm|Chemical Communications]] | rok vydání = 2014 | strany = 9827–9830 | url = https://zenodo.org/record/889879 | doi = 10.1039/C4CC03725A | pmid = 25080328}}</ref>]] |
|||
První dendrimery připravili Fritz Vögtle v roce 1978 divergentní syntézou,<ref>{{Citace periodika | autor1 = E. Buhleier | autor2 = W. Wehner | autor3 = F. Vögtle | titul = "Cascade"- and "Nonskid-Chain-like" Syntheses of Molecular Cavity Topologies | periodikum = [[Synthesis]] | rok vydání = 1978 | strany = 155–158 | doi = 10.1055/s-1978-24702}}</ref> R. G. Denkewalter v roce 1981,<ref>{{US Patent|4289872}} Denkewalter, Robert G., Kolc, Jaroslav, Lukasavage, William J.</ref><ref>Denkewalter, Robert G. et al. (1981) "Macromolecular highly branched homogeneous compound" {{US Patent|4,410,688}}</ref> [[Donald Tomalia]] roku 1983<ref>Tomalia, Donald A. and Dewald, James R. (1983) "Dense star polymers having core, core branches, terminal groups" {{US Patent|4507466}}</ref> a 1985<ref>{{Citace periodika | autoři = D. A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, P. Smith | titul = A New Class of Polymers: Starburst-Dendritic Macromolecules | periodikum = Polymer Journal | rok vydání = 1985 | strany = 117–132 | doi = 10.1295/polymj.17.117}}</ref><ref>{{cite news| url=http://www.thefreelibrary.com/Treelike+molecules+branch+out.-a017817461 | work=Science News | title=Treelike molecules branch out – chemist Donald A. Tomalia synthesized first dendrimer molecule – Chemistry – Brief Article | year=1996}}</ref> a ve stejném roce i [[George R. Newkome]].<ref name=newkome>{{Citace periodika | autor1 = G. R. Newkome | autor2 = Z. Yao | autor3 = G. R. Baker | autor4 = V. K. Gupta | titul = Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol | periodikum = [[The Journal of Organic Chemistry]] | rok vydání = 1985 | strany = 2003–2004 | doi = 10.1295/polymj.17.117}}</ref> |
|||
V roce 1990 vyvinuli [[Craig Hawker]] a [[Jean Fréchet]] konvergentní metodu přípravy.<ref>{{Citace periodika | autor1 = C. J. Hawker | autor2 = J. M. Fréchet | titul = Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules | periodikum = [[Journal of the American Chemical Society]] | rok vydání = 1990 | strany = 7638–7647 | doi = 10.1021/ja00177a027}}</ref> |
|||
== Vlastnosti == |
|||
Dendrimery a dendrony se vyznačují [[disperzita|monodisperzitou]] a obvykle i vysokou symetričností a sféričností molekul. Dendritické molekuly lze přibližně rozdělit na látky s nízkými a vysokými [[molární hmotnost|molárními hmotnostmi]]. Do první kategorie patří dendrimery a dendrony, do druhé [[dendronizované polymery]], hyperrozvětvené polymery a [[polymerové kartáč]]e. |
|||
<!-- The properties of dendrimers are dominated by the [[functional groups]] on the [[Van der Waals surface|molecular surface]], however, there are examples of dendrimers with internal functionality.<ref>{{cite journal | vauthors = Antoni P, Hed Y, Nordberg A, Nyström D, von Holst H, Hult A, Malkoch M | title = Bifunctional dendrimers: from robust synthesis and accelerated one-pot postfunctionalization strategy to potential applications | journal = Angewandte Chemie | volume = 48 | issue = 12 | pages = 2126–30 | year = 2009 | pmid = 19117006 | doi = 10.1002/anie.200804987 }}</ref><ref>{{cite journal | vauthors = McElhanon JR, McGrath DV | title = Toward chiral polyhydroxylated dendrimers. Preparation and chiroptical properties | journal = The Journal of Organic Chemistry | volume = 65 | issue = 11 | pages = 3525–9 | date = June 2000 | pmid = 10843641 | doi = 10.1021/jo000207a }}</ref><ref>{{cite journal | vauthors = Liang CO, Fréchet JM | year = 2005 | title = Incorporation of Functional Guest Molecules into an Internally Functionalizable Dendrimer through Olefin Metathesis| journal = [[Macromolecules (journal)|Macromolecules]] | volume = 38 | issue = 15| pages = 6276–6284 | doi = 10.1021/ma050818a | bibcode = 2005MaMol..38.6276L }}</ref> Dendritic [[Molecular encapsulation|encapsulation]] of functional molecules allows for the isolation of the active site, a structure that mimics that of active sites in biomaterials.<ref>{{cite journal | vauthors = Hecht S, Fréchet JM | title = Dendritic Encapsulation of Function: Applying Nature's Site Isolation Principle from Biomimetics to Materials Science | journal = Angewandte Chemie | volume = 40 | issue = 1 | pages = 74–91 | date = January 2001 | pmid = 11169692 | doi = 10.1002/1521-3773(20010105)40:1<74::AID-ANIE74>3.0.CO;2-C }}</ref><ref>{{cite book| vauthors = Frechet J, Tomalia DA |title=Dendrimers and Other Dendritic Polymers|publisher=John Wiley & Sons|location=New York, NY|date=March 2002|isbn=978-0-471-63850-6}}</ref><ref>{{cite journal | journal = [[Angew. Chem. Int. Ed.]] | year = 1999 | volume = 38 | pages = 884–905 | doi = 10.1002/(SICI)1521-3773(19990401)38:7<884::AID-ANIE884>3.0.CO;2-K | title = Dendrimers: From Design to Application—A Progress Report | issue = 7| vauthors = Fischer M, Vögtle F }}</ref> Also, it is possible to make dendrimers water-soluble, unlike most [[polymers]], by functionalizing their outer shell with charged species or other [[hydrophilic]] groups. Other controllable properties of dendrimers include [[toxicity]], [[crystallinity]], tecto-dendrimer formation, and [[chirality (chemistry)|chirality]].<ref name=review /> |
|||
Dendrimers are also classified by generation, which refers to the number of repeated branching cycles that are performed during its synthesis. For example, if a dendrimer is made by convergent synthesis (see below), and the branching reactions are performed onto the core molecule three times, the resulting dendrimer is considered a third generation dendrimer. Each successive generation results in a dendrimer roughly twice the molecular weight of the previous generation. Higher generation dendrimers also have more exposed functional groups on the surface, which can later be used to customize the dendrimer for a given application.<ref name=holister>{{cite web|url=http://www.sps.aero/Key_ComSpace_Articles/TSA-001_Dendrimers_White%20Paper.pdf |title=Dendrimers: Technology White Papers | vauthors = Holister P, Vas CR, Harper T |date=October 2003 |publisher=Cientifica |access-date=17 March 2010 |url-status=dead |archive-url=https://web.archive.org/web/20110706065720/http://www.sps.aero/Key_ComSpace_Articles/TSA-001_Dendrimers_White%20Paper.pdf |archive-date=6 July 2011 }}</ref> |
|||
==Synthesis== |
|||
[[image:538 Arborol.png|thumb|Synthesis to second generation arborol]] |
|||
One of the first dendrimers, the Newkome dendrimer, was synthesized in 1985. This [[macromolecule]] is also commonly known by the name arborol. The figure outlines the mechanism of the first two generations of arborol through a divergent route (discussed below). The synthesis is started by [[nucleophilic substitution]] of 1-bromopentane by ''triethyl sodiomethanetricarboxylate'' in [[dimethylformamide]] and [[benzene]]. The [[ester]] groups were then [[organic reduction|reduced]] by [[lithium aluminium hydride]] to a [[alcohol|triol]] in a [[protective group|deprotection]] step. Activation of the chain ends was achieved by converting the alcohol groups to [[tosylate]] groups with [[tosyl chloride]] and [[pyridine]]. The tosyl group then served as [[leaving group]]s in another reaction with the tricarboxylate, forming generation two. Further repetition of the two steps leads to higher generations of arborol.<ref name=newkome /> |
|||
[[Poly(amidoamine)]], or PAMAM, is perhaps the most well known dendrimer. The core of PAMAM is a diamine (commonly [[ethylenediamine]]), which is reacted with [[methyl acrylate]], and then another ethylenediamine to make the generation-0 (G-0) PAMAM. Successive reactions create higher generations, which tend to have different properties. Lower generations can be thought of as flexible molecules with no appreciable inner regions, while medium-sized (G-3 or G-4) do have internal space that is essentially separated from the outer shell of the dendrimer. Very large (G-7 and greater) dendrimers can be thought of more like solid particles with very dense surfaces due to the structure of their outer shell. The functional group on the surface of PAMAM dendrimers is ideal for [[click chemistry]], which gives rise to many potential applications.<ref name=bioconj /> |
|||
Dendrimers can be considered to have three major portions: a core, an inner shell, and an outer shell. Ideally, a dendrimer can be synthesized to have different functionality in each of these portions to control properties such as solubility, thermal stability, and attachment of compounds for particular applications. Synthetic processes can also precisely control the size and number of branches on the dendrimer. There are two defined methods of dendrimer synthesis, [[divergent synthesis]] and [[convergent synthesis]]. However, because the actual reactions consist of many steps needed to protect the [[active site]], it is difficult to synthesize dendrimers using either method. This makes dendrimers hard to make and very expensive to purchase. At this time, there are only a few companies that sell dendrimers; [[Polymer Factory Sweden AB]]<ref>Polymer Factory AB, Stockholm, Sweden.[http://www.PolymerFactory.com Polymer Factory]</ref> commercializes biocompatible bis-MPA dendrimers and Dendritech<ref>Dendritech Inc., from Midland, Michigan, USA.[http://www.dendritech.com Dendritech].</ref> is the only kilogram-scale producers of PAMAM dendrimers. NanoSynthons, LLC<ref>[http://www.Nanosynthons.com Home]. NanoSynthons. Retrieved on 2015-09-29.</ref> from Mount Pleasant, Michigan, USA produces PAMAM dendrimers and other proprietary dendrimers. |
|||
===Divergent methods=== |
|||
[[image:538 Divergent synthesis.png|thumb|Schematic of divergent synthesis of dendrimers]] |
|||
The dendrimer is assembled from a multifunctional core, which is extended outward by a series of reactions, commonly a [[Michael reaction]]. Each step of the reaction must be driven to full completion to prevent mistakes in the dendrimer, which can cause trailing generations (some branches are shorter than the others). Such impurities can impact the functionality and symmetry of the dendrimer, but are extremely difficult to purify out because the relative size difference between perfect and imperfect dendrimers is very small.<ref name=holister /> |
|||
===Convergent methods=== |
|||
[[image:538 Convergent synthesis.png|thumb|Schematic of convergent synthesis of dendrimers]] |
|||
Dendrimers are built from small molecules that end up at the surface of the sphere, and reactions proceed inward building inward and are eventually attached to a core. This method makes it much easier to remove impurities and shorter branches along the way, so that the final dendrimer is more monodisperse. However dendrimers made this way are not as large as those made by divergent methods because crowding due to [[steric effects]] along the core is limiting.<ref name=holister /> |
|||
===Click chemistry=== |
|||
[[Image:Dendrimer DA Mullen 1996.svg|thumb|Dendrimer [[Diels-Alder reaction]].<ref>{{cite journal|doi=10.1002/anie.199706311|title=Polyphenylene Dendrimers: From Three-Dimensional to Two-Dimensional Structures|journal=Angewandte Chemie International Edition in English|volume=36|issue=6|pages=631–634|year=1997|last1=Morgenroth|first1=Frank|last2=Reuther|first2=Erik|last3=Müllen|first3=Klaus | name-list-style = vanc }}</ref>]] |
|||
Dendrimers have been prepared via [[click chemistry]], employing [[Diels-Alder reaction]]s,<ref>{{cite journal | vauthors = Franc G, Kakkar AK | title = Diels-Alder "click" chemistry in designing dendritic macromolecules | journal = Chemistry | volume = 15 | issue = 23 | pages = 5630–9 | date = June 2009 | pmid = 19418515 | doi = 10.1002/chem.200900252 }}</ref> thiol-ene and [[thiol-yne reaction]]s <ref>{{cite journal | vauthors = Killops KL, Campos LM, Hawker CJ | title = Robust, efficient, and orthogonal synthesis of dendrimers via thiol-ene "click" chemistry | journal = Journal of the American Chemical Society | volume = 130 | issue = 15 | pages = 5062–4 | date = April 2008 | pmid = 18355008 | doi = 10.1021/ja8006325 | citeseerx = 10.1.1.658.8715 }}</ref> and [[Azide alkyne Huisgen cycloaddition|azide-alkyne reactions]].<ref>{{cite journal | vauthors = Noda K, Minatogawa Y, Higuchi T | title = Effects of hippocampal neurotoxicant, trimethyltin, on corticosterone response to a swim stress and glucocorticoid binding capacity in the hippocampus in rats | journal = The Japanese Journal of Psychiatry and Neurology | volume = 45 | issue = 1 | pages = 107–8 | date = March 1991 | pmid = 1753450 }}</ref><ref>{{cite journal | vauthors = Machaiah JP | title = Changes in macrophage membrane proteins in relation to protein deficiency in rats | journal = Indian Journal of Experimental Biology | volume = 29 | issue = 5 | pages = 463–7 | date = May 1991 | pmid = 1916945 }}</ref><ref>{{cite journal | vauthors = Franc G, Kakkar A | title = Dendrimer design using Cu(I)-catalyzed alkyne-azide "click-chemistry" | journal = Chemical Communications | issue = 42 | pages = 5267–76 | date = November 2008 | pmid = 18985184 | doi = 10.1039/b809870k }}</ref> |
|||
There are ample avenues that can be opened by exploring this chemistry in dendrimer synthesis. |
|||
==Applications== |
|||
Applications of dendrimers typically involve conjugating other chemical species to the dendrimer surface that can function as detecting agents (such as a [[dye]] molecule), affinity [[ligands]], targeting components, [[radioligand]]s, [[Contrast medium|imaging agent]]s, or [[pharmaceutically active compounds]]. Dendrimers have very strong potential for these applications because their structure can lead to [[multivalent]] systems. In other words, one dendrimer molecule has hundreds of possible sites to couple to an active species. Researchers aimed to utilize the hydrophobic environments of the dendritic media to conduct photochemical reactions that generate the products that are synthetically challenged. Carboxylic acid and phenol-terminated water-soluble dendrimers were synthesized to establish their utility in drug delivery as well as conducting chemical reactions in their interiors.<ref>{{cite journal | vauthors = Kaanumalle LS, Ramesh R, Murthy Maddipatla VS, Nithyanandhan J, Jayaraman N, Ramamurthy V | title = Dendrimers as photochemical reaction media. Photochemical behavior of unimolecular and bimolecular reactions in water-soluble dendrimers | journal = The Journal of Organic Chemistry | volume = 70 | issue = 13 | pages = 5062–9 | date = June 2005 | pmid = 15960506 | doi = 10.1021/jo0503254 }}</ref> This might allow researchers to attach both targeting molecules and drug molecules to the same dendrimer, which could reduce negative side effects of medications on healthy cells.<ref name="bioconj">{{cite book|last=Hermanson|first=Greg T.| name-list-style = vanc |title=Bioconjugate Techniques|publisher=Academic Press of Elsevier|location=London|year=2008|edition=2nd|chapter=7|isbn=978-0-12-370501-3}}</ref> |
|||
Dendrimers can also be used as a solubilizing agent. Since their introduction in the mid-1980s, this novel class of dendrimer architecture has been a prime candidate for [[host–guest chemistry]].<ref>{{cite journal | title = Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter | journal = [[Angew. Chem. Int. Ed. Engl.]] | year = 1990 | pages = 138–175 | volume = 29 | doi = 10.1002/anie.199001381 | issue = 2| last1 = Tomalia | first1 = Donald A. | last2 = Naylor | first2 = Adel M. | last3 = Goddard | first3 = William A. | name-list-style = vanc }}</ref> Dendrimers with hydrophobic core and hydrophilic periphery have shown to exhibit micelle-like behavior and have container properties in solution.<ref>{{cite journal | vauthors = Fréchet JM | title = Functional polymers and dendrimers: reactivity, molecular architecture, and interfacial energy | journal = Science | volume = 263 | issue = 5154 | pages = 1710–5 | date = March 1994 | pmid = 8134834 | doi = 10.1126/science.8134834 | bibcode = 1994Sci...263.1710F }}</ref> The use of dendrimers as unimolecular micelles was proposed by Newkome in 1985.<ref>{{cite journal | vauthors = Liu M, Kono K, Fréchet JM | title = Water-soluble dendritic unimolecular micelles: their potential as drug delivery agents | journal = Journal of Controlled Release | volume = 65 | issue = 1–2 | pages = 121–31 | date = March 2000 | pmid = 10699276 | doi = 10.1016/s0168-3659(99)00245-x }}</ref> This analogy highlighted the utility of dendrimers as solubilizing agents.<ref>{{cite journal | title = Micelles Part 1. Cascade molecules: a new approach to micelles, A-arborol | journal = [[J. Org. Chem.]] | year = 1985 | pages = 155–158 | volume = 50 | issue = 11 | doi=10.1021/jo00211a052| last1 = Newkome | first1 = George R. | last2 = Yao | first2 = Zhongqi | last3 = Baker | first3 = Gregory R. | last4 = Gupta | first4 = Vinod K. | name-list-style = vanc }}</ref> The majority of drugs available in pharmaceutical industry are hydrophobic in nature and this property in particular creates major formulation problems. This drawback of drugs can be ameliorated by dendrimeric scaffolding, which can be used to encapsulate as well as to solubilize the drugs because of the capability of such scaffolds to participate in extensive hydrogen bonding with water.<ref>{{cite journal | title = Synthesis, characterisation and guest-host properties of inverted unimolecular micelles | vauthors = Stevelmens S, Hest JC, Jansen JF, Boxtel DA, de Bravander-van den B, Miejer EW | journal = [[J Am Chem Soc]] | year = 1996 | pages = 7398–7399 | volume = 118 | doi = 10.1021/ja954207h | issue = 31|url=https://research.tue.nl/nl/publications/synthesis-characterization-and-guesthost-properties-of-inverted-unimolecular-dendritic-micelles(947d0f26-7215-44a3-a4ad-49fba0d24282).html }}</ref><ref>{{cite journal | vauthors = Gupta U, Agashe HB, Asthana A, Jain NK | title = Dendrimers: novel polymeric nanoarchitectures for solubility enhancement | journal = Biomacromolecules | volume = 7 | issue = 3 | pages = 649–58 | date = March 2006 | pmid = 16529394 | doi = 10.1021/bm050802s }}</ref><ref>{{cite journal | vauthors = Thomas TP, Majoros IJ, Kotlyar A, Kukowska-Latallo JF, Bielinska A, Myc A, Baker JR | title = Targeting and inhibition of cell growth by an engineered dendritic nanodevice | journal = Journal of Medicinal Chemistry | volume = 48 | issue = 11 | pages = 3729–35 | date = June 2005 | pmid = 15916424 | doi = 10.1021/jm040187v }}</ref><ref>{{cite journal | vauthors = Bhadra D, Bhadra S, Jain P, Jain NK | title = Pegnology: a review of PEG-ylated systems | journal = Die Pharmazie | volume = 57 | issue = 1 | pages = 5–29 | date = January 2002 | pmid = 11836932 }}</ref><ref>{{cite journal | vauthors = Asthana A, Chauhan AS, Diwan PV, Jain NK | title = Poly(amidoamine) (PAMAM) dendritic nanostructures for controlled site-specific delivery of acidic anti-inflammatory active ingredient | journal = AAPS PharmSciTech | volume = 6 | issue = 3 | pages = E536-42 | date = October 2005 | pmid = 16354015 | pmc = 2750401 | doi = 10.1208/pt060367 }}</ref><ref>{{cite journal | vauthors = Bhadra D, Bhadra S, Jain S, Jain NK | title = A PEGylated dendritic nanoparticulate carrier of fluorouracil | journal = International Journal of Pharmaceutics | volume = 257 | issue = 1–2 | pages = 111–24 | date = May 2003 | pmid = 12711167 | doi = 10.1016/s0378-5173(03)00132-7 }}</ref> Dendrimer labs throughout the planet are persistently trying to manipulate dendrimer's solubilizing trait, in their way to explore dendrimer as drug delivery <ref>{{cite journal | vauthors = Khopade AJ, Caruso F, Tripathi P, Nagaich S, Jain NK | title = Effect of dendrimer on entrapment and release of bioactive from liposomes | journal = International Journal of Pharmaceutics | volume = 232 | issue = 1–2 | pages = 157–62 | date = January 2002 | pmid = 11790499 | doi = 10.1016/S0378-5173(01)00901-2 }}</ref><ref>{{cite journal | vauthors = Prajapati RN, Tekade RK, Gupta U, Gajbhiye V, Jain NK | title = Dendimer-mediated solubilization, formulation development and in vitro-in vivo assessment of piroxicam | journal = Molecular Pharmaceutics | volume = 6 | issue = 3 | pages = 940–50 | year = 2009 | pmid = 19231841 | doi = 10.1021/mp8002489 }}</ref> and target specific carrier.<ref>{{cite journal | vauthors = Chauhan AS, Sridevi S, Chalasani KB, Jain AK, Jain SK, Jain NK, Diwan PV | title = Dendrimer-mediated transdermal delivery: enhanced bioavailability of indomethacin | journal = Journal of Controlled Release | volume = 90 | issue = 3 | pages = 335–43 | date = July 2003 | pmid = 12880700 | doi = 10.1016/s0168-3659(03)00200-1 }}</ref><ref>{{cite journal | vauthors = Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR | display-authors = 6 | title = Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer | journal = Cancer Research | volume = 65 | issue = 12 | pages = 5317–24 | date = June 2005 | pmid = 15958579 | doi = 10.1158/0008-5472.can-04-3921 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Quintana A, Raczka E, Piehler L, Lee I, Myc A, Majoros I, Patri AK, Thomas T, Mulé J, Baker JR | display-authors = 6 | title = Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor | journal = Pharmaceutical Research | volume = 19 | issue = 9 | pages = 1310–6 | date = September 2002 | pmid = 12403067 | doi = 10.1023/a:1020398624602 | url = https://deepblue.lib.umich.edu/bitstream/2027.42/41493/1/11095_2004_Article_378868.pdf | hdl = 2027.42/41493 | s2cid = 9444825 }}</ref> |
|||
For dendrimers to be able to be used in pharmaceutical applications, they must surmount the required regulatory [[Drug development|hurdles]] to reach market. One dendrimer scaffold designed to achieve this is the Poly Ethoxy Ethyl Glycinamide (PEE-G) dendrimer.<ref>{{cite journal | vauthors = Toms S, Carnachan SM, Hermans IF, Johnson KD, Khan AA, O'Hagan SE, Tang CW, Rendle PM | display-authors = 6 | title = Poly Ethoxy Ethyl Glycinamide (PEE-G) Dendrimers: Dendrimers Specifically Designed for Pharmaceutical Applications | journal = ChemMedChem | volume = 11 | issue = 15 | pages = 1583–6 | date = August 2016 | pmid = 27390296 | doi = 10.1002/cmdc.201600270 | s2cid = 5007374 }}</ref><ref>{{Cite web|last=GlycoSyn|url=http://www.glycofinechem.glycosyn.com/collections/dendrimers|title=PEE-G Dendrimers}}</ref> This dendrimer scaffold has been designed and shown to have high [[High-performance liquid chromatography|HPLC]] purity, stability, aqueous solubility and low inherent toxicity. |
|||
===Drug delivery=== |
|||
[[File:538 Gene delivery.png|thumb|Scheme of a G-5 PAMAM dendrimer conjugated to both a dye molecule and a strand of DNA.]] |
|||
Approaches for delivering unaltered natural products using polymeric carriers is of widespread interest. Dendrimers have been explored for the encapsulation of [[hydrophobic]] compounds and for the delivery of anticancer drugs. The physical characteristics of dendrimers, including their monodispersity, water solubility, encapsulation ability, and large number of functionalizable peripheral groups make these [[macromolecule]]s appropriate candidates for drug delivery vehicles. |
|||
==== Role of dendrimer chemical modifications in drug delivery ==== |
|||
Dendrimers are particularly versatile drug delivery devices due to the wide range of chemical modifications that can be made to increase in vivo suitability and allow for site-specific targeted drug delivery. |
|||
Drug attachment to the dendrimer may be accomplished by (1) a covalent attachment or conjugation to the external surface of the dendrimer forming a dendrimer prodrug, (2) ionic coordination to charged outer functional groups, or (3) micelle-like encapsulation of a drug via a dendrimer-drug supramolecular assembly.<ref>{{cite journal | vauthors = Morgan MT, Nakanishi Y, Kroll DJ, Griset AP, Carnahan MA, Wathier M, Oberlies NH, Manikumar G, Wani MC, Grinstaff MW | display-authors = 6 | title = Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro | journal = Cancer Research | volume = 66 | issue = 24 | pages = 11913–21 | date = December 2006 | pmid = 17178889 | doi = 10.1158/0008-5472.CAN-06-2066 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Tekade RK, Dutta T, Gajbhiye V, Jain NK | title = Exploring dendrimer towards dual drug delivery: pH responsive simultaneous drug-release kinetics | journal = Journal of Microencapsulation | volume = 26 | issue = 4 | pages = 287–96 | date = June 2009 | pmid = 18791906 | doi = 10.1080/02652040802312572 | s2cid = 44523215 }}</ref> In the case of a dendrimer prodrug structure, linking of a drug to a dendrimer may be direct or linker-mediated depending on desired release kinetics. Such a linker may be pH-sensitive, enzyme catalyzed, or a disulfide bridge. The wide range of terminal functional groups available for dendrimers allows for many different types of linker chemistries, providing yet another tunable component on the system. Key parameters to consider for linker chemistry are (1) release mechanism upon arrival to the target site, whether that be within the cell or in a certain organ system, (2) drug-dendrimer spacing so as to prevent lipophilic drugs from folding into the dendrimer, and (3) linker degradability and post-release trace modifications on drugs.<ref>{{cite journal | vauthors = Leong NJ, Mehta D, McLeod VM, Kelly BD, Pathak R, Owen DJ, Porter CJ, Kaminskas LM | display-authors = 6 | title = Doxorubicin Conjugation and Drug Linker Chemistry Alter the Intravenous and Pulmonary Pharmacokinetics of a PEGylated Generation 4 Polylysine Dendrimer in Rats | journal = Journal of Pharmaceutical Sciences | volume = 107 | issue = 9 | pages = 2509–2513 | date = September 2018 | pmid = 29852134 | doi = 10.1016/j.xphs.2018.05.013 | url = https://espace.library.uq.edu.au/view/UQ:e652bb4/UQe652bb4_OA.pdf }}</ref><ref>{{cite journal | vauthors = da Silva Santos S, Igne Ferreira E, Giarolla J | title = Dendrimer Prodrugs | journal = Molecules | volume = 21 | issue = 6 | pages = 686 | date = May 2016 | pmid = 27258239 | pmc = 6274429 | doi = 10.3390/molecules21060686 }}</ref> |
|||
[[Polyethylene glycol]] (PEG) is a common modification for dendrimers to modify their surface charge and circulation time. Surface charge can influence the interactions of dendrimers with biological systems, such as amine-terminal modified dendrimers which have a propensity to interact with cell membranes with anionic charge. Certain in vivo studies have shown polycationic dendrimers to be cytotoxic through membrane permeabilization, a phenomenon that could be partially mitigated via addition of PEGylation caps on amine groups, resulting in lower cytotoxicity and lower red blood cell hemolysis.<ref name="Dendrimer pharmacokinetics: the eff">{{cite journal | vauthors = Kaminskas LM, Boyd BJ, Porter CJ | title = Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties | journal = Nanomedicine | volume = 6 | issue = 6 | pages = 1063–84 | date = August 2011 | pmid = 21955077 | doi = 10.2217/nnm.11.67 }}</ref><ref name=":0">{{cite journal | vauthors = Luong D, Kesharwani P, Deshmukh R, Mohd Amin MC, Gupta U, Greish K, Iyer AK | title = PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery | journal = Acta Biomaterialia | volume = 43 | pages = 14–29 | date = October 2016 | pmid = 27422195 | doi = 10.1016/j.actbio.2016.07.015 }}</ref> Additionally, studies have found that PEGylation of dendrimers results in higher drug loading, slower drug release, longer circulation times in vivo, and lower toxicity in comparison to counterparts without PEG modifications.<ref name=":1">{{cite journal | vauthors = Singh P, Gupta U, Asthana A, Jain NK | title = Folate and folate-PEG-PAMAM dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice | journal = Bioconjugate Chemistry | volume = 19 | issue = 11 | pages = 2239–52 | date = November 2008 | pmid = 18950215 | doi = 10.1021/bc800125u }}</ref><ref name=":0" /> |
|||
Numerous targeting moieties have been used to modify dendrimer biodistribution and allow for targeting to specific organs. For example, folate receptors are overexpressed in tumor cells and are therefore promising targets for localized drug delivery of [[chemotherapeutics]]. Folic acid conjugation to PAMAM dendrimers has been shown to increase targeting and decrease off-target toxicity while maintaining on-target cytotoxicity of chemotherapeutics such as [[methotrexate]], in mouse models of cancer.<ref name=":1" /><ref>{{cite journal | vauthors = Majoros IJ, Williams CR, Becker A, Baker JR | title = Methotrexate delivery via folate targeted dendrimer-based nanotherapeutic platform | journal = Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology | volume = 1 | issue = 5 | pages = 502–10 | date = September 2009 | pmid = 20049813 | pmc = 2944777 | doi = 10.1002/wnan.37}}</ref> |
|||
Antibody-mediated targeting of dendrimers to cell targets has also shown promise for targeted drug delivery. As [[epidermal growth factor receptor]]s (EGFRs) are often overexpressed in brain tumors, EGFRs are a convenient target for site-specific drug delivery. The delivery of boron to cancerous cells is important for effective neutron capture therapy, a cancer treatment which requires a large concentration of boron in cancerous cells and a low concentration in healthy cells. A boronated dendrimer conjugated with a monoclonal antibody drug that targets EGFRs was used in rats to successfully deliver boron to cancerous cells.<ref>{{cite journal | vauthors = Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MJ, Fenstermaker RA | title = Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy | journal = Bioconjugate Chemistry | volume = 15 | issue = 1 | pages = 185–94 | date = January 2004 | pmid = 14733599 | doi = 10.1021/bc0341674}}</ref> |
|||
Modifying [[nanoparticle]] dendrimers with [[peptide]]s has also been successful for targeted destruction of colorectal ([[HCT116 cells|HCT-116]]) cancer cells in a co-culture scenario. [[Target peptide|Targeting peptides]] can be used to achieve site- or cell-specific delivery, and it has been shown that these peptides increase in targeting specificity when paired with dendrimers. Specifically, gemcitabine-loaded YIGSR-CMCht/PAMAM, a unique kind of dendrimer nanoparticle, induces a targeted mortality on these cancer cells. This is performed via selective interaction of the dendrimer with [[laminin]] receptors. Peptide dendrimers may be employed in the future to precisely target cancer cells and deliver chemotherapeutic agents.<ref>{{cite journal| vauthors = Carvalho MR, Carvalho CR, Maia FR, Caballero D, Kundu SC, Reis RL, Oliveira JM |date= November 2019 |title=Peptide‐Modified Dendrimer Nanoparticles for Targeted Therapy of Colorectal Cancer |journal=Advanced Therapeutics |volume=2|issue=11|pages=1900132|doi=10.1002/adtp.201900132|hdl= 1822/61410 |s2cid= 203135854 |issn=2366-3987|hdl-access=free}}</ref> |
|||
The cellular uptake mechanism of dendrimers can also be tuned using chemical targeting modifications. Non-modified PAMAM-G4 dendrimer is taken up into activated microglia by fluid phase endocytosis. Conversely, mannose modification of hydroxyl PAMAM-G4 dendrimers was able to change the mechanism of internalization to mannose-receptor (CD206) mediated endocytosis. Additionally, mannose modification was able to change the [[biodistribution]] in the rest of the body in rabbits.<ref>{{cite journal | vauthors = Sharma A, Porterfield JE, Smith E, Sharma R, Kannan S, Kannan RM | title = Effect of mannose targeting of hydroxyl PAMAM dendrimers on cellular and organ biodistribution in a neonatal brain injury model | journal = Journal of Controlled Release | volume = 283 | pages = 175–189 | date = August 2018 | pmid = 29883694 | pmc = 6091673 | doi = 10.1016/j.jconrel.2018.06.003}}</ref> |
|||
==== Pharmacokinetics and pharmacodynamics ==== |
|||
Dendrimers have the potential to completely change the [[Pharmacokinetics|pharmacokinetic]] and [[Pharmacodynamics|pharmacodynamic]] (PK/PD) profiles of a drug. As carriers, the PK/PD is no longer determined by the drug itself but by the dendrimer’s localization, drug release, and dendrimer excretion. [[ADME]] properties are very highly tunable by varying dendrimer size, structure, and surface characteristics. While G9 dendrimers biodistribute very heavily to the liver and spleen, G6 dendrimers tend to biodistribute more broadly. As molecular weight increases, urinary clearance and plasma clearance decrease while terminal half-life increases.<ref name="Dendrimer pharmacokinetics: the eff"/> |
|||
==== Routes of delivery ==== |
|||
To increase patient compliance with prescribed treatment, delivery of drugs orally is often preferred to other routes of drug administration. However oral [[bioavailability]] of many drugs tends to be very low. Dendrimers can be used to increase the solubility and stability of orally-administered drugs and increase drug penetration through the intestinal membrane.<ref>{{cite journal | vauthors = Csaba N, Garcia-Fuentes M, Alonso MJ | title = The performance of nanocarriers for transmucosal drug delivery | journal = Expert Opinion on Drug Delivery | volume = 3 | issue = 4 | pages = 463–78 | date = July 2006 | pmid = 16822222 | doi = 10.1517/17425247.3.4.463 | s2cid = 13056713}}</ref> The bioavailability of PAMAM dendrimers conjugated to a chemotherapeutic has been studied in mice; it was found that around 9% of dendrimer administered orally was found intact in circulation and that minimal dendrimer degradation occurred in the gut.<ref>{{cite journal | vauthors = Thiagarajan G, Sadekar S, Greish K, Ray A, Ghandehari H | title = Evidence of oral translocation of anionic G6.5 dendrimers in mice | journal = Molecular Pharmaceutics | volume = 10 | issue = 3 | pages = 988–98 | date = March 2013 | pmid = 23286733 | pmc = 3715149 | doi = 10.1021/mp300436c}}</ref> |
|||
Intravenous dendrimer delivery shows promise as gene vectors to deliver genes to various organs in the body, and even tumors. One study found that through intravenous injection, a combination of PPI dendrimers and gene complexes resulted in gene expression in the liver, and another study showed that a similar injection regressed the growth of tumors in observed animals.<ref>{{cite journal | vauthors = Dufès C, Uchegbu IF, Schätzlein AG | title = Dendrimers in gene delivery | journal = Advanced Drug Delivery Reviews | volume = 57 | issue = 15 | pages = 2177–202 | date = December 2005 | pmid = 16310284 | doi = 10.1016/j.addr.2005.09.017 | url = https://strathprints.strath.ac.uk/7822/1/Dufesetal2005review.pdf}}</ref><ref>{{cite journal | vauthors = Dufès C, Keith WN, Bilsland A, Proutski I, Uchegbu IF, Schätzlein AG | title = Synthetic anticancer gene medicine exploits intrinsic antitumor activity of cationic vector to cure established tumors | journal = Cancer Research | volume = 65 | issue = 18 | pages = 8079–84 | date = September 2005 | pmid = 16166279 | doi = 10.1158/0008-5472.CAN-04-4402 | doi-access = free}}</ref> |
|||
The primary obstacle to transdermal drug delivery is the epidermis. Hydrophobic drugs have a very difficult time penetrating the skin layer, as they partition heavily into skin oils. Recently, PAMAM dendrimers have been used as delivery vehicles for [[Nonsteroidal anti-inflammatory drug|NSAIDS]] to increase hydrophilicity, allowing greater drug penetration.<ref>{{cite journal | vauthors = Cheng Y, Man N, Xu T, Fu R, Wang X, Wang X, Wen L | title = Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (PAMAM) dendrimers | journal = Journal of Pharmaceutical Sciences | volume = 96 | issue = 3 | pages = 595–602 | date = March 2007 | pmid = 17094130 | doi = 10.1002/jps.20745}}</ref> These modifications act as polymeric transdermal enhancers allowing drugs to more easily penetrate the skin barrier. |
|||
Dendrimers may also act as new [[Ophthalmic drug administration|ophthalmic]] vehicles for drug delivery, which are different from the polymers currently used for this purpose. A study by Vanndamme and Bobeck used PAMAM dendrimers as ophthalmic delivery vehicles in rabbits for two model drugs and measured the ocular residence time of this delivery to be comparable and in some cases greater than current [[bioadhesive]] polymers used in ocular delivery.<ref>{{cite journal | vauthors = Vandamme TF, Brobeck L | title = Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide | journal = Journal of Controlled Release | volume = 102 | issue = 1 | pages = 23–38 | date = January 2005 | pmid = 15653131 | doi = 10.1016/j.jconrel.2004.09.015}}</ref> This result indicates that administered drugs were more active and had increased bioavailability when delivered via dendrimers than their free-drug counterparts. Additionally, photo-curable, drug-eluting dendrimer-hyaluronic acid hydrogels have been used as corneal sutures applied directly to the eye. These hydrogel sutures have shown efficacy as a medical device in rabbit models that surpasses traditional sutures and minimizes corneal scarring.<ref>{{cite journal | vauthors = Xu Q, Kambhampati SP, Kannan RM | title = Nanotechnology approaches for ocular drug delivery | journal = Middle East African Journal of Ophthalmology | volume = 20 | issue = 1 | pages = 26–37 | date = 2013 | pmid = 23580849 | pmc = 3617524 | doi = 10.4103/0974-9233.106384}}</ref> |
|||
==== Brain drug delivery ==== |
|||
Dendrimer drug delivery has also shown major promise as a potential solution for many traditionally difficult drug delivery problems. In the case of drug delivery to the brain, dendrimers are able to take advantage of the [[Enhanced permeability and retention effect|EPR effect]] and [[Blood–brain barrier|blood-brain barrier]] (BBB) impairment to cross the BBB effectively in vivo. For example, hydroxyl-terminated PAMAM dendrimers possess an intrinsic targeting ability to inflamed [[macrophage]]s in the brain, verified using fluorescently labeled neutral generation dendrimers in a rabbit model of [[cerebral palsy]].<ref name=":2">{{cite journal | vauthors = Dai H, Navath RS, Balakrishnan B, Guru BR, Mishra MK, Romero R, Kannan RM, Kannan S | display-authors = 6 | title = Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration | journal = Nanomedicine | volume = 5 | issue = 9 | pages = 1317–29 | date = November 2010 | pmid = 21128716 | pmc = 3095441 | doi = 10.2217/nnm.10.89}}</ref> This intrinsic targeting has enabled drug delivery in a variety of conditions, ranging from cerebral palsy and other neuroinflammatory disorders to traumatic brain injury and hypothermic circulatory arrest, across a variety of animal models ranging from mice and rabbits to canines.<ref>{{cite journal | vauthors = Kannan G, Kambhampati SP, Kudchadkar SR | title = Effect of anesthetics on microglial activation and nanoparticle uptake: Implications for drug delivery in traumatic brain injury | journal = Journal of Controlled Release | volume = 263 | pages = 192–199 | date = October 2017 | pmid = 28336376 | doi = 10.1016/j.jconrel.2017.03.032 | s2cid = 8652471}}</ref><ref>{{cite journal | vauthors = Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM | display-authors = 6 | title = Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model | journal = Science Translational Medicine | volume = 4 | issue = 130 | pages = 130ra46 | date = April 2012 | pmid = 22517883 | pmc = 3492056 | doi = 10.1126/scitranslmed.3003162}}</ref><ref>{{cite journal | vauthors = Mishra MK, Beaty CA, Lesniak WG, Kambhampati SP, Zhang F, Wilson MA, Blue ME, Troncoso JC, Kannan S, Johnston MV, Baumgartner WA, Kannan RM | display-authors = 6 | title = Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest | journal = ACS Nano | volume = 8 | issue = 3 | pages = 2134–47 | date = March 2014 | pmid = 24499315 | pmc = 4004292 | doi = 10.1021/nn404872e}}</ref> Dendrimer uptake into the brain correlates with severity of inflammation and BBB impairment and it is believed that the BBB impairment is the key driving factor allowing dendrimer penetration.<ref>{{cite journal | vauthors = Nance E, Kambhampati SP, Smith ES, Zhang Z, Zhang F, Singh S, Johnston MV, Kannan RM, Blue ME, Kannan S | display-authors = 6 | title = Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome | journal = Journal of Neuroinflammation | volume = 14 | issue = 1 | pages = 252 | date = December 2017 | pmid = 29258545 | pmc = 5735803 | doi = 10.1186/s12974-017-1004-5}}</ref><ref name=":2" /> Localization is heavily skewed towards activated [[microglia]]. Dendrimer-conjugated N-acetyl cysteine has shown efficacy in vivo as an anti-inflammatory at more than 1000-fold lower dose than free drug on a drug basis, reversing the phenotype of cerebral palsy, [[Rett syndrome]], [[macular degeneration]] and other inflammatory diseases.<ref name=":2" /> |
|||
==== Clinical trials ==== |
|||
Starpharma, an Australian pharmaceutical company, has multiple products that have either already been approved for use or are in the clinical trial phase. SPL7013, also known as astodrimer sodium, is a hyperbranched polymer used in Starpharma’s VivaGel line of pharmaceuticals that is currently approved to treat bacterial vaginosis and prevent the spread of HIV, HPV, and HSV in Europe, Southeast Asia, Japan, Canada, and Australia. Due to SPL7013’s broad antiviral action, it has recently been tested by the company as a potential drug to treat SARS-CoV-2. The company states preliminary in-vitro studies show high efficacy in preventing SARS-CoV-2 infection in cells.<ref>{{Cite web|url=https://themarketherald.com.au/starpharma-asxspl-compound-shows-activity-against-coronavirus-2020-04-16/,%20https://themarketherald.com.au/starpharma-asxspl-compound-shows-activity-against-coronavirus-2020-04-16/|title=Starpharma (ASX:SPL) compound shows activity against coronavirus - The Market Herald|date=2020-04-16|website=themarketherald.com.au|language=en-US|access-date=2020-04-30}}</ref> |
|||
===Gene delivery and transfection=== |
|||
The ability to deliver pieces of [[DNA]] to the required parts of a cell includes many challenges. Current research is being performed to find ways to use dendrimers to traffic genes into cells without damaging or deactivating the DNA. To maintain the activity of DNA during dehydration, the dendrimer/DNA complexes were encapsulated in a water-soluble polymer, and then deposited on or sandwiched in functional polymer films with a fast degradation rate to mediate gene [[transfection]]. Based on this method, PAMAM dendrimer/DNA complexes were used to encapsulate functional biodegradable polymer films for substratemediated gene delivery. Research has shown that the fast-degrading functional polymer has great potential for localized transfection.<ref>{{cite journal | vauthors = Fu HL, Cheng SX, Zhang XZ, Zhuo RX | title = Dendrimer/DNA complexes encapsulated functional biodegradable polymer for substrate-mediated gene delivery | journal = The Journal of Gene Medicine | volume = 10 | issue = 12 | pages = 1334–42 | date = December 2008 | pmid = 18816481 | doi = 10.1002/jgm.1258 | s2cid = 46011138 }}</ref><ref>{{cite journal | vauthors = Fu HL, Cheng SX, Zhang XZ, Zhuo RX | title = Dendrimer/DNA complexes encapsulated in a water soluble polymer and supported on fast degrading star poly(DL-lactide) for localized gene delivery | journal = Journal of Controlled Release | volume = 124 | issue = 3 | pages = 181–8 | date = December 2007 | pmid = 17900738 | doi = 10.1016/j.jconrel.2007.08.031 }}</ref><ref>{{cite journal | vauthors = Dutta T, Garg M, Jain NK | title = Poly(propyleneimine) dendrimer and dendrosome mediated genetic immunization against hepatitis B | journal = Vaccine | volume = 26 | issue = 27–28 | pages = 3389–94 | date = June 2008 | pmid = 18511160 | doi = 10.1016/j.vaccine.2008.04.058 }}</ref> |
|||
===Sensors=== |
|||
Dendrimers have potential applications in [[sensor]]s. Studied systems include [[proton]] or [[pH]] sensors using poly(propylene imine),<ref>{{cite journal| vauthors = Fernandes EG, Vieira NC, de Queiroz AA, Guimaraes FE, Zucolotto V |year=2010|title=Immobilization of Poly(propylene imine) Dendrimer/Nickel Phthalocyanine as Nanostructured Multilayer Films To Be Used as Gate Membranes for SEGFET pH Sensors |journal=Journal of Physical Chemistry C|volume=114|issue=14|pages=6478–6483|doi=10.1021/jp9106052}}</ref> cadmium-sulfide/polypropylenimine tetrahexacontaamine dendrimer composites to detect [[fluorescence]] signal [[quenching]],<ref>{{cite journal | vauthors = Campos BB, Algarra M, Esteves da Silva JC | title = Fluorescent properties of a hybrid cadmium sulfide-dendrimer nanocomposite and its quenching with nitromethane | journal = Journal of Fluorescence | volume = 20 | issue = 1 | pages = 143–51 | date = January 2010 | pmid = 19728051 | doi = 10.1007/s10895-009-0532-5 | s2cid = 10846628 }}</ref> and poly(propylenamine) first and second generation dendrimers for metal [[cation]] [[photodetection]]<ref>{{cite journal | vauthors = Grabchev I, Staneva D, Chovelon JM |year=2010|title=Photophysical investigations on the sensor potential of novel, poly(propylenamine) dendrimers modified with 1,8-naphthalimide units|journal=Dyes and Pigments|volume=85|issue=3|pages=189–193|doi=10.1016/j.dyepig.2009.10.023}}</ref> amongst others. Research in this field is vast and ongoing due to the potential for multiple detection and binding sites in dendritic structures. |
|||
===Nanoparticles=== |
|||
Dendrimers also are used in the synthesis of [[monodisperse]] metallic nanoparticles. Poly(amidoamide), or PAMAM, dendrimers are utilized for their tertiary amine groups at the branching points within the dendrimer. Metal ions are introduced to an aqueous dendrimer solution and the metal ions form a complex with the lone pair of electrons present at the tertiary amines. After complexation, the ions are reduced to their zerovalent states to form a nanoparticle that is encapsulated within the dendrimer. These nanoparticles range in width from 1.5 to 10 nanometers and are called [[dendrimer-encapsulated nanoparticles]].<ref>{{cite journal | vauthors = Scott RW, Wilson OM, Crooks RM | title = Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles | journal = The Journal of Physical Chemistry B | volume = 109 | issue = 2 | pages = 692–704 | date = January 2005 | pmid = 16866429 | doi = 10.1021/jp0469665 }}</ref> |
|||
=== Other Applications === |
|||
Given the widespread use of pesticides, herbicides and insecticides in modern farming, dendrimers are also being used by companies to help improve the delivery of agrochemicals to enable healthier plant growth and to help fight plant diseases.<ref>{{Cite web|url=http://www.labonline.com.au/content/life-scientist/news/dendrimer-technology-licensed-for-herbicide-701112647|title=Dendrimer technology licensed for herbicide|website=www.labonline.com.au|access-date=2016-09-25}}</ref> |
|||
Dendrimers are also being investigated for use as [[blood substitutes]]. Their steric bulk surrounding a [[heme]]-mimetic centre significantly slows degradation compared to free heme,<ref>{{cite journal|vauthors=Twyman LJ, Ge Y|date=April 2006|title=Porphyrin cored hyperbranched polymers as heme protein models|journal=Chemical Communications|issue=15|pages=1658–60|doi=10.1039/b600831n|pmid=16583011}}</ref><ref>{{cite journal|vauthors=Twyman LJ, Ellis A, Gittins PJ|date=January 2012|title=Pyridine encapsulated hyperbranched polymers as mimetic models of haeme containing proteins, that also provide interesting and unusual porphyrin-ligand geometries|journal=Chemical Communications|volume=48|issue=1|pages=154–6|doi=10.1039/c1cc14396d|pmid=22039580}}</ref> and prevents the [[cytotoxicity]] exhibited by free heme. |
|||
Dendritic functional polymer polyamidoamine (PAMAM) is used to prepare core shell structure i.e. microcapsules and utilized in formulation of self-healing coatings of conventional <ref>Tatiya, Pyus D., et al. "Novel polyurea microcapsules using dendritic functional monomer: synthesis, characterization, and its use in self-healing and anticorrosive polyurethane coatings." Industrial & Engineering Chemistry Research 52.4 (2013): 1562-1570.</ref> and renewable origins.<ref>Chaudhari, Ashok B., et al. "Polyurethane prepared from neem oil polyesteramides for self-healing anticorrosive coatings." Industrial & Engineering Chemistry Research 52.30 (2013): 10189-10197.</ref> |
|||
== See also == |
|||
{{Commons category|Dendrimers}} |
|||
* [[Dendronized polymer]] |
|||
* [[Metallodendrimer]] |
|||
* [[Ferrocene-containing dendrimers]] |
|||
== References == --> |
|||
[[Kategorie:Supramolekulární chemie]] |
[[Kategorie:Supramolekulární chemie]] |
||
Verze z 23. 3. 2021, 19:15

Dendrimery (také se používají označení arboroly, dendrony a kaskádové molekuly) jsou vysoce uspořádané polymery s rozvětvenými molekulami.[1][2]
Molekuly dendrimerů jsou obvykle souměrné podle svých středů a často zaujímají kulovitý tvar. Většinou obsahují chemicky dostupnou funkční skupinu, nazývanou ohnisko nebo jádro. Rozdíly mezi dendrimery a dendrony jsou zobrazeny výše; tyto pojmy se však často zaměňují.[3]

První dendrimery připravili Fritz Vögtle v roce 1978 divergentní syntézou,[6] R. G. Denkewalter v roce 1981,[7][8] Donald Tomalia roku 1983[9] a 1985[10][11] a ve stejném roce i George R. Newkome.[12]
V roce 1990 vyvinuli Craig Hawker a Jean Fréchet konvergentní metodu přípravy.[13]
Vlastnosti
Dendrimery a dendrony se vyznačují monodisperzitou a obvykle i vysokou symetričností a sféričností molekul. Dendritické molekuly lze přibližně rozdělit na látky s nízkými a vysokými molárními hmotnostmi. Do první kategorie patří dendrimery a dendrony, do druhé dendronizované polymery, hyperrozvětvené polymery a polymerové kartáče.
- ↑ D. Astruc; E. Boisselier; C. Ornelas. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chemical Reviews. 2010, s. 1857–1959. DOI 10.1021/cr900327d. PMID 20356105.
- ↑ Vögtle, Fritz / Richardt, Gabriele / Werner, Nicole Dendrimer Chemistry Concepts, Syntheses, Properties, Applications 2009 ISBN 3-527-32066-0
- ↑ B. K. Nanjwade; H. M. Bechra; G. K. Derkar; F. V. Manvi; V. K. Nanjwade. Dendrimers: emerging polymers for drug-delivery systems. European Journal of Pharmaceutical Sciences. 2009, s. 185–196. DOI 10.1016/j.ejps.2009.07.008. PMID 19646528.
- ↑ Single-Crystal Structures of Polyphenylene Dendrimers. Chemistry: A European Journal. 2002, s. 3858–3864. DOI 10.1002/1521-3765(20020902)8:17<3858::AID-CHEM3858>3.0.CO;2-5.
- ↑ B. E. Hirsch; S. Lee; B. Qiao; C. H. Chen; K. P. McDonald; S. L. Tait; A. H. Flood. Anion-induced dimerization of 5-fold symmetric cyanostars in 3D crystalline solids and 2D self-assembled crystals. Chemical Communications. 2014, s. 9827–9830. Dostupné online. DOI 10.1039/C4CC03725A. PMID 25080328.
- ↑ E. Buhleier; W. Wehner; F. Vögtle. "Cascade"- and "Nonskid-Chain-like" Syntheses of Molecular Cavity Topologies. Synthesis. 1978, s. 155–158. DOI 10.1055/s-1978-24702.
- ↑ Šablona:US Patent Denkewalter, Robert G., Kolc, Jaroslav, Lukasavage, William J.
- ↑ Denkewalter, Robert G. et al. (1981) "Macromolecular highly branched homogeneous compound" Šablona:US Patent
- ↑ Tomalia, Donald A. and Dewald, James R. (1983) "Dense star polymers having core, core branches, terminal groups" Šablona:US Patent
- ↑ A New Class of Polymers: Starburst-Dendritic Macromolecules. Polymer Journal. 1985, s. 117–132. DOI 10.1295/polymj.17.117.
- ↑ Treelike molecules branch out – chemist Donald A. Tomalia synthesized first dendrimer molecule – Chemistry – Brief Article. Science News. 1996. Dostupné online.
- ↑ G. R. Newkome; Z. Yao; G. R. Baker; V. K. Gupta. Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol. The Journal of Organic Chemistry. 1985, s. 2003–2004. DOI 10.1295/polymj.17.117.
- ↑ C. J. Hawker; J. M. Fréchet. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. Journal of the American Chemical Society. 1990, s. 7638–7647. DOI 10.1021/ja00177a027.
